Several studies have assessed visual contrast detection in patients with schizophrenia, but the findings among these studies have not been consistent with each other. Some studies have reported higher visual contrast detection thresholds in patients than in nonpsychiatric comparison subjects
(1–
4), and another study found that schizophrenic patients did not differ in visual contrast detection from comparison subjects
(5). In a preliminary report, Kéri and colleagues
(6) noted that visual contrast detection thresholds were significantly lower in schizophrenic patients than in nonpsychiatric comparison subjects. The explanation for these disparate results may reside in the type of antipsychotic drugs the patients were receiving. Most of the patients included in the studies that reported poor performance were receiving depot antipsychotic medications, which are typical neuroleptics. Most of the patients Chen et al.
(5) tested were receiving atypical antipsychotic medications.
Because of the apparent link between contrast sensitivity and drug regimen, we address here whether antipsychotic treatment type affects visual contrast detection by comparing the contrast thresholds of patients with schizophrenia receiving typical, atypical, or no antipsychotic medication with those of healthy subjects.
The issue of medication effects on visual contrast detection assumes importance for a broader understanding of sensory processes in schizophrenia when one recognizes that dopamine release affects aspects of visual processing
(7–
11). For example, dopamine, through D
2-mediated mechanisms, increases the inhibitory effect of the surrounding areas of a retinal neural unit’s receptive field, leading to an enhancement of visual contrast detection
(12,
13). Indeed, dopamine plays a central role in the ability to detect visual contrast. Dopamine deficiency, induced experimentally in monkeys treated with the neurotoxin MPTP and in pathological hypodopaminergic conditions such as Parkinson’s disease, results in decreased visual contrast sensitivity
(11) and affects the contrast-elicited electroretinograms
(13).
In the aforementioned studies of visual contrast sensitivity in schizophrenia, with the exception of the study by Kéri et al.
(6), all of the patients were medicated at the time of testing, and all had been medicated for a significant period of time during their treatment. These antipsychotic medications are known to bind to dopamine receptors, principally the D
2 receptor family. The older typical antipsychotic medications, such as chlorpromazine and haloperidol, carry with them unwanted side effects, such as extrapyramidal symptoms and elevated prolactin secretion. The newer so-called atypical antipsychotic medications, such as clozapine and quetiapine, generally do not produce these effects. In a recent discussion of the mechanism of action of antipsychotic compounds, Seeman
(14) showed that although both atypical and typical drugs block the D
2 receptor, the atypical antipsychotics, such as clozapine and quetiapine, have very short occupancy periods with relatively steep declines even to insignificance within 48 hours, whereas the typical antipsychotics, such as haloperidol, maintain essentially undiminished occupancy for days. The rapid release of the atypical antipsychotics allows endogenous dopamine to reoccupy the receptor. The slow release of the typical antipsychotics maintains the blockade of the receptor, making dopamine less available.
Because dopamine modulation is implicated both in visual contrast detection and the treatment of schizophrenia, it is possible that therapeutically administered compounds that affect dopamine receptor dynamics would affect visual contrast detection in patients. We hypothesized that patients receiving typical neuroleptics, which are accompanied by tight and relatively long-lasting D
2 occupancy, would show visual contrast thresholds that were significantly higher than those of healthy subjects. Patients receiving atypical neuroleptics, which are accompanied by loose and relatively short lasting D
2 occupancy, would show visual contrast detection thresholds that did not differ significantly from those of healthy subjects. Inasmuch as the major issue in this study involves a state-related effect of medication on visual contrast detection, we wanted to see whether the pharmacological compounds in question affected a variable that has previously been shown to be a trait characteristic of a schizophrenic disposition: velocity discrimination. That is, in an earlier study we reported that schizophrenia patients and a proportion of their first-degree relatives showed motion detection thresholds that were higher than those of nonpsychiatric comparison subjects
(5), whereas visual contrast detection thresholds were normal in both the patients and in the relatives, who were clinically unaffected and had never been treated with antipsychotic medication. In the present study, we examined the visual contrast thresholds of a new group of relatives of schizophrenia patients with the expectation that their visual contrast detection thresholds would be comparable to those of the healthy group. Normal visual contrast detection levels in the clinically unaffected relatives would strengthen the hypothesis that visual contrast detection variations are state-related.
Method
Subjects
Forty-five schizophrenic patients participated in this study. Thirty-nine were receiving antipsychotic medication. Eight patients were being treated with a typical antipsychotic drug (haloperidol: N=1; fluphenazine: N=2; trifluoperazine: N=1; perphenazine: N=2; perphenazine and fluphenazine: N=1; loxapine: N=1). Twenty-five patients were being treated with an atypical antipsychotic drug, some of whom received two such drugs (clozapine: N=19; olanzapine: N=12; risperidone: N=8; quetiapine: N=3), and six received both types of drugs. The six remaining patients were receiving no antipsychotic drugs; three of the six were receiving an antidepressant (SSRI) medication, and the other three were receiving no psychoactive drugs. For all patients, medication choice was determined by the treating psychiatrist independent of this study.
All patients had been discharged during the preceding year from a private psychiatric hospital and met DSM-IV criteria for schizophrenia (N=12) or schizoaffective disorder, mixed type (N=33). Consensus diagnoses were made independently and blind to the experimental design and results by a team of four experienced clinicians on the basis of a review of the Structured Clinical Interview for DSM-IV (SCID)
(15) conducted by trained interviewers and an evaluation of all available hospital records. The average score on the Brief Psychiatric Rating Scale (BPRS)
(16) for the patients was 42.4 (SD=13.9).
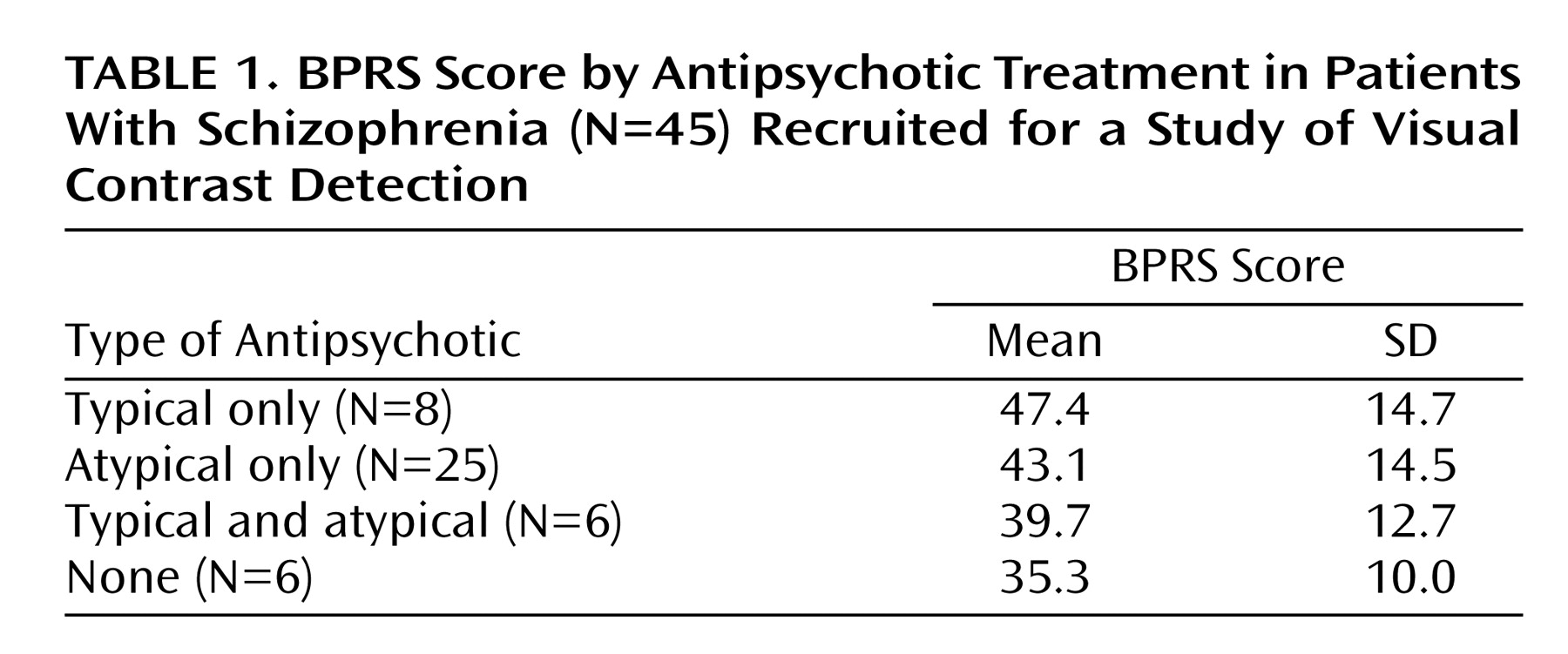
Table 1 presents BPRS data for the patients according to antipsychotic treatment received. The average duration of illness was 16.0 years (SD=9.2), indicating that these patients were chronically ill and in various degrees of partial remission.
Thirty-nine nonpsychiatric comparison subjects and 24 unaffected first-degree relatives of the schizophrenia patients also participated in this study. None of these participants met DSM-IV criteria for a psychotic condition (lifetime) or for schizotypal, schizoid, or paranoid personality disorder, according to both the SCID and the Structured Interview for Schizotypy
(17). None of these normal subjects and relatives had ever received any antipsychotic medication.
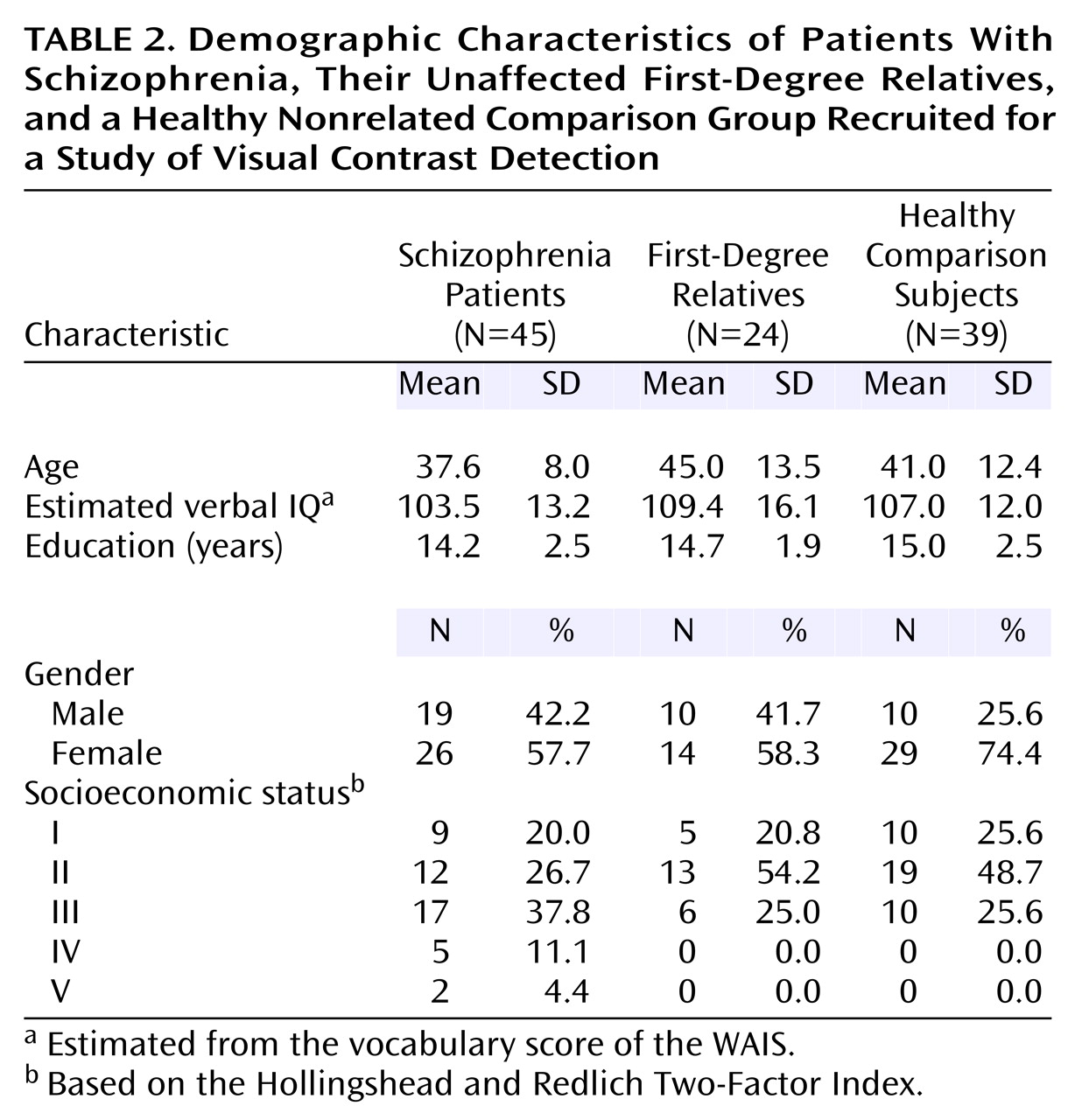
The groups did not differ significantly in terms of any of the demographic characteristics (
Table 2). The McLean Hospital and Harvard University institutional review boards approved the protocol for this study. Before being tested, all participants gave written informed consent in accord with the respective institutional review board guidelines.
Stimuli
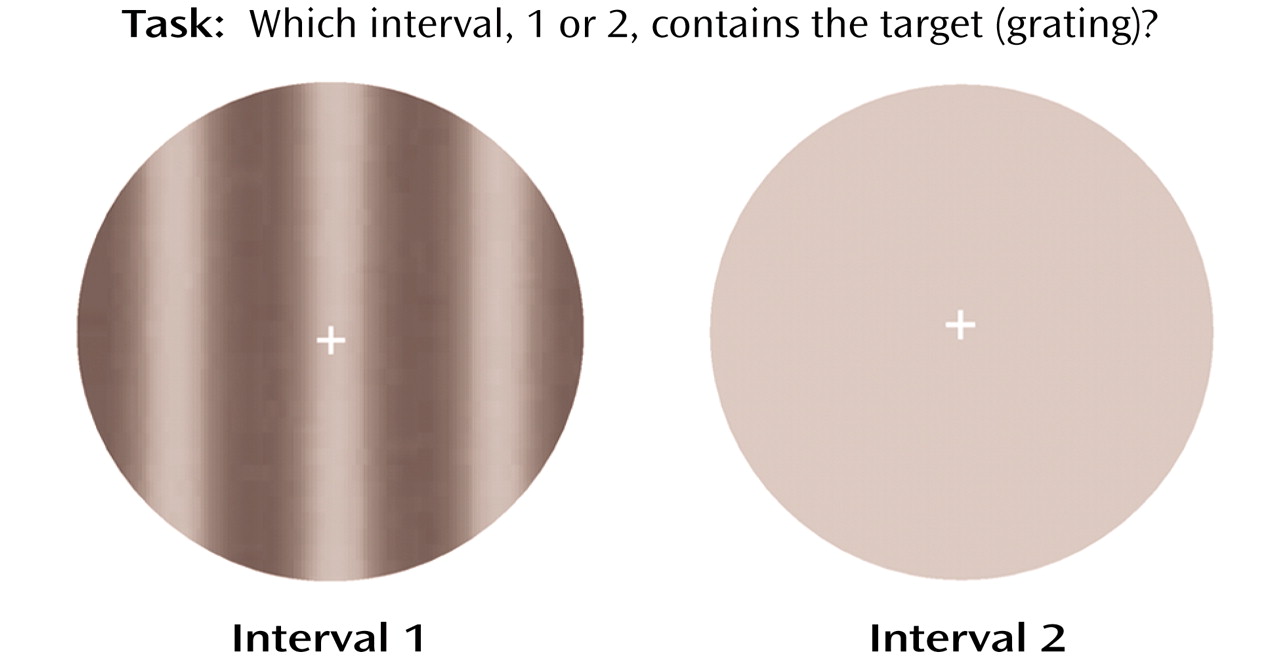
The target for assessing visual contrast detection was a vertical grating (
Figure 1). The spatial luminance distribution of the grating was sinusoidal, and the spatial frequency was 0.5 cycles/degree. The temporal modulation was set at 5 Hz, which yielded target movement of 10˚/sec, either to the right or to the left. This combination of spatial and temporal frequencies made the grating optimal for visual contrast detection
(18). The target was presented within a circular window with a diameter of 10˚ of visual angle. Target contrast is defined as C=(L
max – L
min)/(L
max + L
min), where C=contrast, L
max=maximal luminance, and L
min=minimal luminance. A fixation cross was provided at the center of the field.
Procedure
The subjects’ task was to indicate which of the two successively presented circles (first or second) in a trial contained the target. During each trial there were two successively presented intervals, one containing the target and the other a blank area whose luminance level was the same as that of the average of the target. The target could appear in either the first or the second interval, randomized across the trials. Subjects made a judgment immediately after each trial by pressing one of two designated keys (1 or 2). The time between two intervals in a trial was 500 msec. Intertrial intervals were 1000 msec. Each grating was presented on the screen for 300 msec. The dependent measure was the minimum amount of contrast necessary to distinguish the gratings from the blank area at 79% accuracy level, a standard typically used in psychophysical studies
(19). Contrast level of the gratings was set initially at 1.5%, a level adequate for all participants to perform the task. The level of contrast varied from trial to trial according to a two-alternative, forced-choice staircase method (1-up-3-down). Specifically, the contrast was decreased by 5% of the current level if three correct responses were made in a row and was increased by 5% of the current level if an incorrect response was made. Twelve reversals of staircase direction terminated an experimental session. The contrast levels at all reversals, except for the first one, were averaged to produce a visual contrast detection threshold.
Detailed instructions and practice trials for the task were administered before formal data collection. All subjects completed all trials.
Results
The schizophrenia group as a whole, and not divided according to medication, had a higher visual contrast detection threshold (mean=0.0036, SD=0.0039) than did the healthy subjects (mean=0.0025, SD=0.0016) (t=2.04, df=82, p<0.05), which indicated poorer contrast sensitivity. Most schizophrenic patients, however, showed visual contrast detection thresholds that were well within the range of those of the normal subjects. The effect size of the group difference was small (0.71).
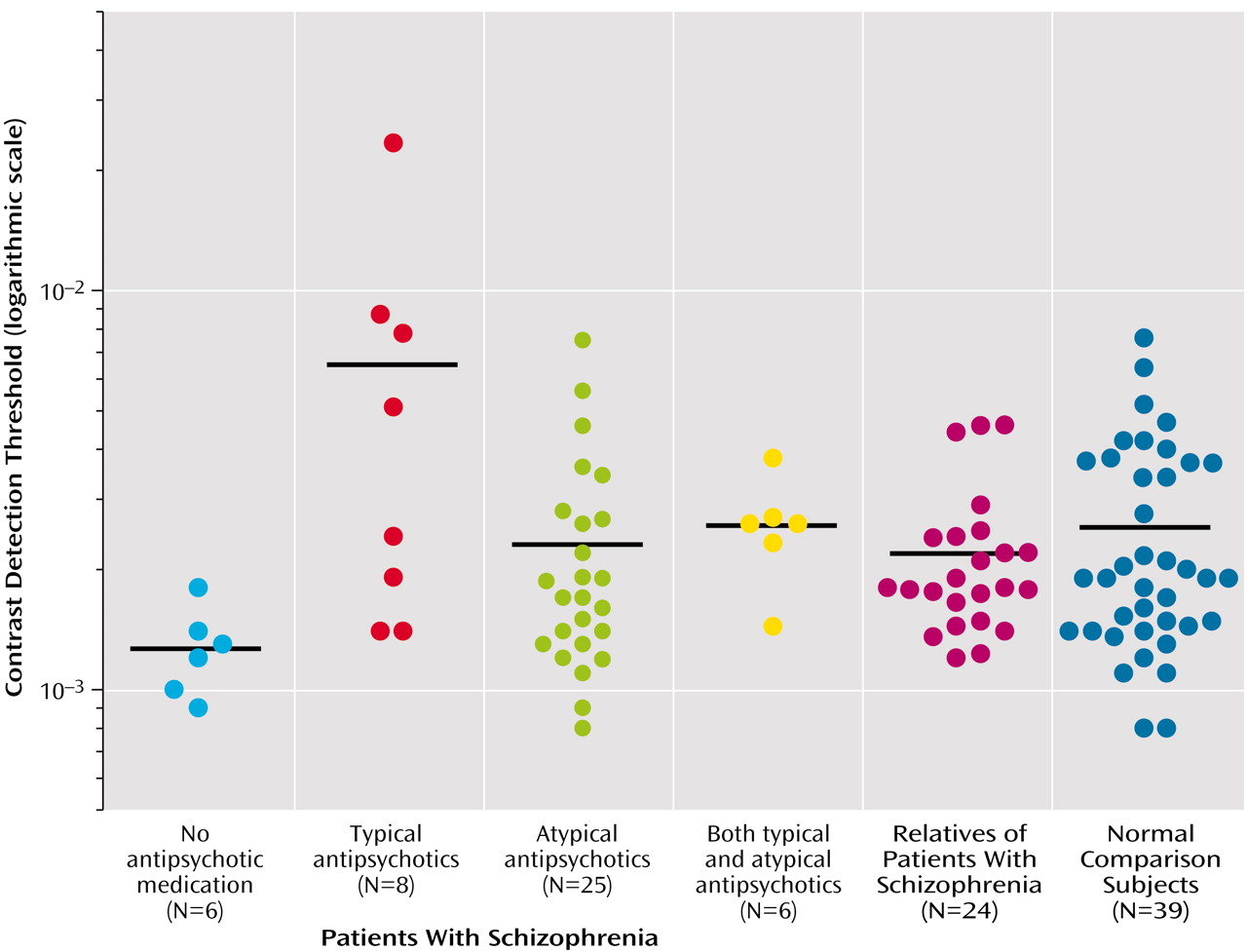
When we classified the patients by the type of antipsychotic medication they received (
Figure 2), it became clear that the average between-group difference reflected the significantly higher visual contrast detection threshold of patients receiving typical antipsychotic drugs. The group receiving typical antipsychotic drugs had significantly higher visual contrast detection thresholds (mean=0.008, SD=0.008) than did the nonpsychiatric comparison subjects (mean=0.0025, SD=0.0016) (t=4.01, df=45, p<0.001; effect size=3.5) and the patients receiving atypical antipsychotics (mean=0.003, SD=0.002) (t=2.79, df=31, p<0.01; effect size=2.72). It is also noteworthy that the visual contrast detection threshold of the group receiving atypical antipsychotic drugs did not differ significantly from that of the healthy subjects (t=0.99, df=62, p>0.10). There were no differences with respect to visual contrast detection threshold between patients with conditions diagnosed as schizophrenia and those as schizoaffective.
The visual contrast detection threshold of the six patients who were receiving no antipsychotic medication (mean=0.0013, SD=0.0004) was significantly lower than that of the healthy subjects (p=0.01, Wilcoxon test; effect size=1.03). Among these six unmedicated patients, three were receiving SSRI medication. The average visual contrast detection threshold for those receiving SSRIs (0.0013) was almost identical to the average of those not receiving SSRIs (0.0012).
The visual contrast detection threshold of the six patients receiving both atypical and typical antipsychotic drugs (mean=0.00267, SD=0.0008) was not significantly different from those receiving only atypical antipsychotic medication, those receiving typical antipsychotic medication, or the healthy subjects.
The visual contrast detection threshold for the group of first-degree relatives of patients with schizophrenia (mean=0.0022, SD=0.001) was not significantly different from the healthy subjects. We also examined visual contrast detection data from 16 relatives who had participated in an earlier study of motion discrimination
(5). Although this subgroup of relatives showed impaired velocity discrimination, comparable to that of the schizophrenic patients, their visual contrast detection thresholds were not different from those of the healthy group in that study. We here compared their visual contrast detection thresholds with their previously obtained motion discrimination thresholds and found no significant correlation between these two tasks (r=0.37, df=15, p>0.10).
There was no significant group difference in BPRS scores, a measure of illness severity, between the patients taking typical antipsychotic drugs and those taking atypical antipsychotic drugs (t=0.48, df=31, p>0.10). The visual contrast detection thresholds and the BPRS scores were essentially uncorrelated within the entire patient group (r=0.02, df=43, p>0.10). It is, however, not entirely possible to reject the idea that clinical state plays a role in these results, inasmuch as 1) the patients receiving typical antipsychotics were the most clinically impaired according to BPRS ratings and had the highest visual contrast detection thresholds, 2) those receiving atypical antipsychotics had somewhat better BPRS scores and also better visual contrast detection thresholds, and 3) the six patients receiving no antipsychotic medication had BPRS scores that showed the least clinical impairment, and they had the lowest visual contrast detection thresholds. When, however, we held the BPRS score constant by conducting an analysis of covariance using the GLM procedure of SAS, with BPRS as the covariate, the results remained unchanged.
Discussion
We observed that the visual contrast detection threshold was significantly lower in the six unmedicated patients than in the healthy subjects and in all of the medicated patients. These results are consistent with data reported by Kéri et al.
(6). With medications, the visual contrast detection thresholds increased in the following patient group order: those receiving atypical antipsychotics, those receiving atypical antipsychotics with the addition of typical antipsychotics, and those receiving typical antipsychotics alone. The visual contrast detection thresholds of patients receiving atypical antipsychotics and the first-degree relatives were approximately equal to those of healthy comparison subjects.
Generalizability of Findings
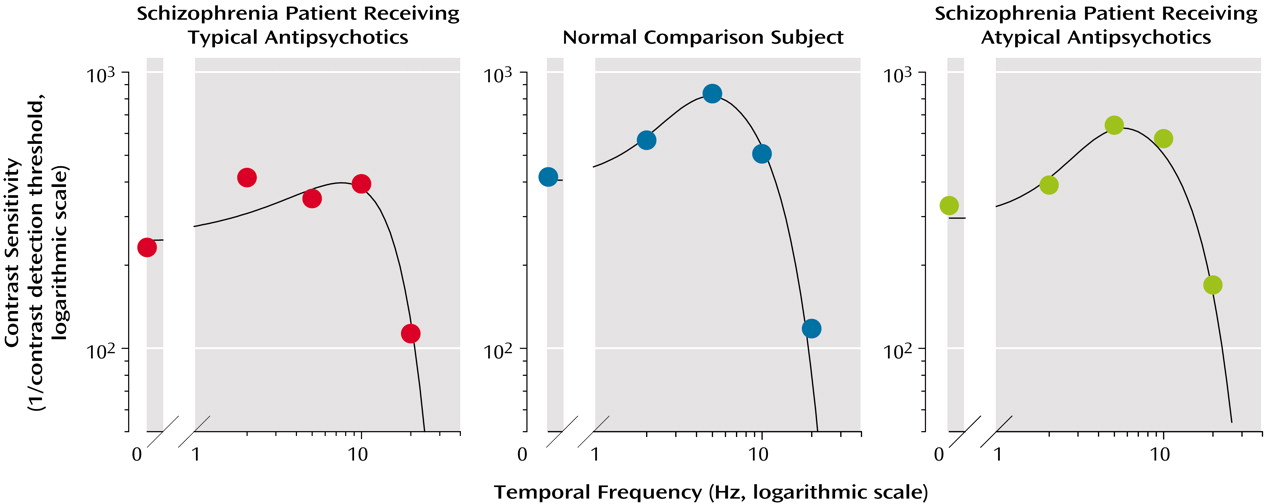
In the present study, we used a single grating with a temporal frequency of 5 Hz, but we believe that the results are generalizable to a wider range of temporal frequencies. We randomly selected one member of each of the three groups (one patient receiving typical antipsychotic medication, one receiving atypical medication, and one healthy subject) and tested their visual contrast detection thresholds over a range of five temporal frequencies (0, 2, 5, 10, and 20 Hz). For each subject, we constructed a contrast sensitivity function that allows one to view the contrast sensitivity (expressed as the reciprocal of the visual contrast detection threshold, on a logarithmic scale) as a function of the temporal frequency (
Figure 3). The patient receiving a typical antipsychotic drug showed a lower peak magnitude of the contrast sensitivity function than did the healthy subject.
The shape of the contrast sensitivity function was also broader (or less tuned) in the patient receiving typical antipsychotic drug than in the healthy subject. On the other hand, the peak magnitude and the bandwidth of the contrast sensitivity function of the patient receiving an atypical antipsychotic drug were close to those of the healthy subject (
Figure 3). These results are consistent with those obtained at 5 Hz and support the finding that the typical antipsychotic drugs raised visual contrast detection thresholds (decreased contrast sensitivity) over a range of temporal frequencies and that the atypical drugs tended to normalize the contrast thresholds.
The Role of Dopamine
Although there may be several pharmacological explanations for these results (such as differences in the regional distribution of the two classes of drugs, with typical antipsychotics mainly occupying the basal ganglia and atypical antipsychotics the frontal cortex; also the relatively greater activity of serotonergic, histaminic, and adrenergic processes seen with atypical antipsychotics), we prefer an explanation that implicates the role of dopamine in both visual contrast detection and in the pharmacological action of the therapeutic compounds used in the treatment of the patients studied here.
Perception of contrast is a response generated early in the visual processing system. At this early stage of visual processing, the response is more or less an undifferentiated one and is without the nuances of color, form, and movement, qualities that accrue later in the visual perceptual process. The detection of contrast begins in the retina, where the result of a visual stimulus is the release of dopamine. Although other neurotransmitters are involved in retinal activity, it is dopamine that is found to modulate detection by keeping the neural activity of retinal cells at the center and the surrounding area in appropriate balance.
The retina relays the information to the lateral geniculate nucleus and then to the primary visual cortex. As Bodis-Wollner
(11) has shown in Parkinson’s disease, a hypodopaminergic disorder, a decrease in dopamine could decrease the inhibition of neural activity in the surrounding retinal cells, resulting in a reduction in contrast sensitivity. And indeed, visual contrast detection thresholds have been shown to be abnormally high in Parkinson’s patients. On the other hand, an increase in endogenous dopamine could lead to an enhancement of the distinction in neural activity between the center and the surrounding retinal cells and thus to increased contrast sensitivity.
Data on the psychopharmacologic action of antipsychotic drugs indicate that all effective antipsychotic drugs block D
2 receptors, even though they may also act on other dopamine and nondopamine receptors. According to Seeman
(14, p. 28), of the five types of dopamine receptors, “only the D
2 receptor is blocked by antipsychotic drugs in direct relation to their clinical antipsychotic potencies.” The atypical drugs bind loosely to the D
2 receptors and are released rapidly, allowing endogenous dopamine to reoccupy the D
2 receptor. The typical antipsychotic drugs, with their long occupancy and tight binding to the D
2 receptors, prevent reoccupancy by endogenous dopamine. This difference between the two classes of antipsychotic drugs may be responsible for the extent to which visual contrast detection is affected. This interpretation is also consistent with the significantly lower than normal visual contrast detection thresholds of the unmedicated patients, who may be in a hyperdopaminergic state in the absence of antipsychotic drug treatment. This group would presumably have the highest amount of endogenous dopamine available at receptors to enhance the distinction in neural activity between the center and the surrounding retinal cells and hence their visual contrast detection. In light of the small size of this group, replication is required. We note, however, that the large effect sizes of the differences between the typical and atypical antipsychotic groups and between the group receiving no medication and the other patient groups as well as the healthy subjects encourage confidence that these differences are valid.
The visual contrast detection threshold of the six patients receiving both typical and atypical drugs was closer to that of the group receiving atypical antipsychotics than that of the group receiving typical antipsychotics and was indistinguishable from that of the healthy group. This result is consistent with the observations by Kalkman et al.
(20) and by Seeman and Tallerico
(21) that the combination of a typical and an atypical drug elicits more of an atypical antipsychotic drug pattern, perhaps by diluting the long-term blocking action of the typical antipsychotics.
The role of dopamine in regulation of cognition has been investigated. Among the most influential of these studies is the work by Goldman-Rakic and her colleagues reporting that too much or too little dopamine impairs working memory in monkeys
(22,
23). Here we report on the role of dopamine in sensory processes and show a similar modulation of visual information by dopamine in schizophrenia.
Contrast Sensitivity and Clinical Symptoms of Schizophrenia
Many readers may recall that Bleuler’s description of schizophrenia included hypersensitivity to the environment, with a tendency to register many events of no intrinsic interest. He attributed these phenomena to an attentional, rather than a sensory, dysfunction, believing this hyperresponsiveness reflected an inability to focus attentional resources so that almost everything is registered. In his opinion, perceptual and sensory processes were intact but there was a breakdown of the “selective inhibitory function of attention,” permitting consciousness to be “flooded with an undifferentiated mass of incoming sensory data transmitted from the environment via the sense organs”
(24, pp. 56–58). In the 1960s, several self-reports of unmedicated patients with schizophrenia appeared that depicted personal events consistent with keenly sensitive contrast experiences in both the visual and auditory spheres. For example, note the following excerpt from McGhie and Chapman
(25): “Not only the color of things fascinates me but all sorts of little things, like markings in the surface pick up my attention too.” A telling self-report quoted by McGhie
(26) is also relevant: “My senses were sharpened. I became fascinated by the little insignificant things around me—sights and sounds possessed a keenness that I had never experienced before—my senses were sharpened.”
We propose that the dynamics of dopamine in regulating visual contrast detection provides a reasonable physiological explanation for these characteristic phenomena of an increased sensitivity that may accompany schizophrenia in its unmedicated state. The unmedicated state was characteristic of most patients with schizophrenia prior to the early 1960s, and possibly as a result, contrast sensitivity could have been high, producing an unusual intensity to visual and auditory stimuli.
Trait and State Factors
We have previously reported that deficient velocity processing is implicated in the smooth pursuit eye movement dysfunctions that have been found not only in patients with schizophrenia but also in a large proportion of their clinically unaffected first-degree relatives
(27–
29). We found that the processing of velocity signals during target motion was also impaired in patients with schizophrenia and in a significant proportion of their unaffected first-degree relatives. We further found that the velocity detection impairment was significantly related to both the initiation and the maintenance phases of smooth pursuit eye movements
(5,
30). There appeared to be no effects of antipsychotic medication of either type on velocity perception.
Two aspects of these earlier reports merit attention here. First, within the group of patients with schizophrenia, contrast and velocity discrimination are dissociated such that antipsychotic drugs affect visual contrast detection but not velocity detection. Further, whereas in unmedicated patients, the visual contrast detection threshold is below that of the healthy group, it rises to normal or higher levels with the administration of antipsychotic medication. The clinically asymptomatic relatives showed normal contrast thresholds even though their velocity discrimination threshold was raised. These data are consistent with the view that regards velocity discrimination, like smooth pursuit eye tracking dysfunctions, as a cofamilial trait, whereas the low visual contrast detection threshold of unmedicated patients appears to be an accompaniment of the clinical disease process of schizophrenia. The contrast sensitivity differences that appear with the different classes of antipsychotic drugs can be readily accounted for by their varying dopamine-blocking effects.