Route of Administration
Routes of administration affect the pharmacokinetic properties, which in turn affect the reinforcing effects of stimulant drugs. Two primary pharmacokinetic properties are relevant for relating serum concentration of methylphenidate to its therapeutic use and abuse: 1) the time to reach maximum concentration (T
max), which is related to the absorption and distribution of the drug, and 2) the time required for the concentration to drop by 50% from the peak level (T
1/2), which is related to the metabolism and excretion of the drug. T
max (rise time) differs dramatically for intravenous and oral dosing, but T
1/2 (fall time) is about the same for these two routes
(17).
The time course of behavioral effects has been documented in studies addressing abuse as well as clinical use of methylphenidate. In abuse, the primary behavioral effects investigated are self-reported drug effects (i.e., the time course for the perception of being “high” as well as for craving and drug liking); in clinical use, the primary behavioral effects are the reductions of symptoms of ADHD (i.e., the time course of the decrease in hyperactivity, inattention, and impulsivity). This section discusses how the pharmacokinetic properties of stimulant drugs affect their behavioral effects.
Temporal Course of Clinical Effects
In children with ADHD, only one pharmacokinetic study comparing intravenous and oral administration of methylphenidate has been reported
(17). Despite dramatic differences in T
max (almost instantaneous for intravenous administration but about 1.5 to 2 hours for oral administration because of the delays in reaching the bloodstream imposed by absorption from the stomach and intestine), this study documented about the same T
1/2 (about 2 hours) for intravenous and oral administration.
After an oral dose, the maximum behavioral effect occurs when the serum concentration reaches its maximum (near T
max) and then declines so that when the serum concentration has dropped about 50% about 2–3 hours later (about T
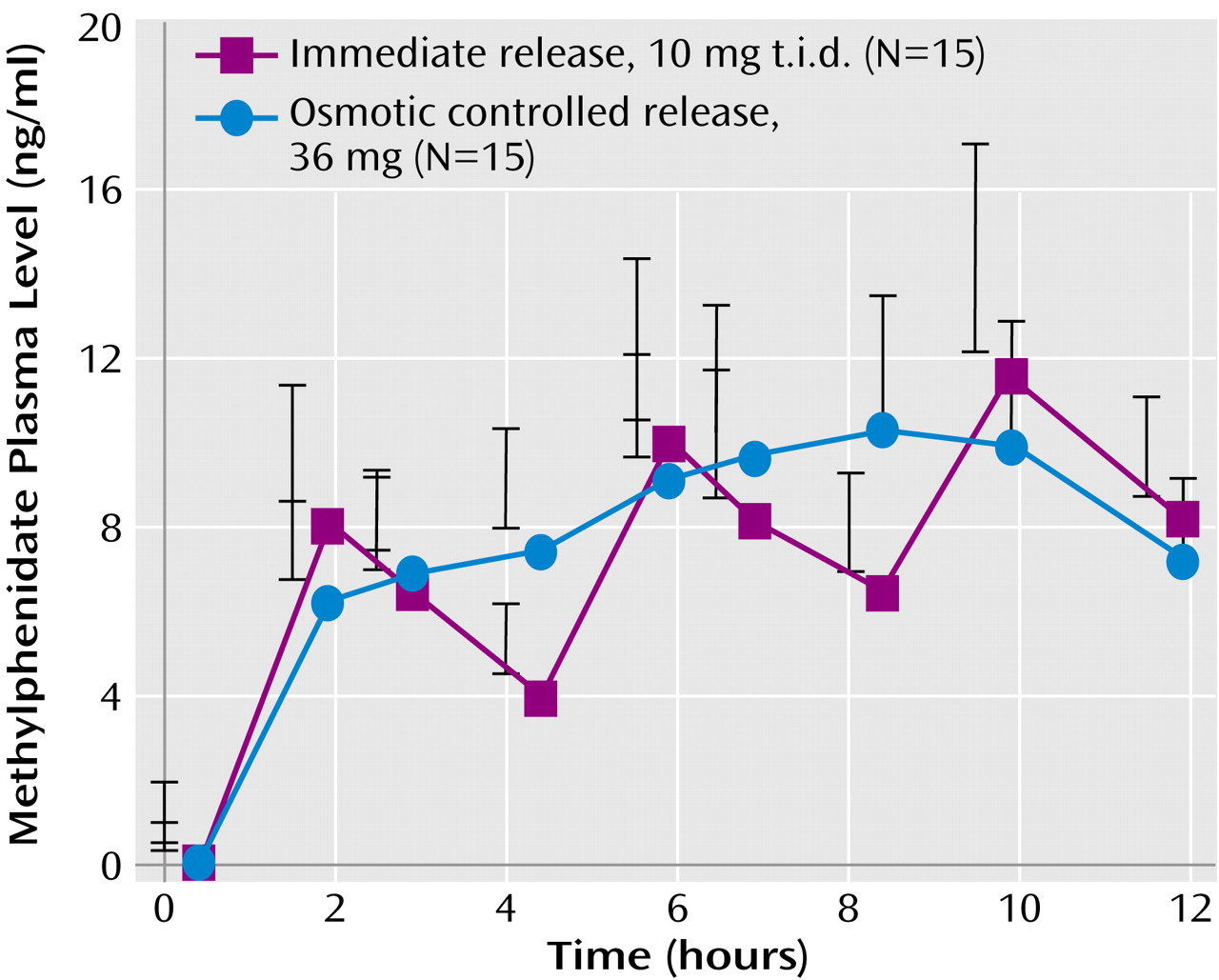
1/2 for methylphenidate), another dose is required to reestablish clinical efficacy. To achieve the 8–12-hour duration of efficacy desired for the clinical treatment of ADHD, dosing two or three times a day is required. For children weighing about 30 kg, a 10-mg t.i.d. regimen of immediate-release methylphenidate (about 0.3 mg/kg per administration) is expected to produce a series of peaks and troughs in plasma concentrations between 4 ng/ml (at the first trough) and 12 ng/ml (at the third peak) (
Figure 2).
For children in school, dosing two or three times a day requires an administration of a controlled Schedule II drug at school. This public administration (often with children lining up at the school office at noon) is inconvenient, costly, and stigmatizing, which created a need for effective sustained-release formulations of methylphenidate that could be administered once a day. However, the first-generation sustained-release formulation, developed and approved for use over 20 years ago, was never well accepted in clinical practice, probably because the wax-matrix delivery system produced a nonoptimal pharmacokinetic profile with a much longer Tmax (about 4 hours) and only a slightly longer T1/2.
Recently, new information about the pharmacokinetic/behavioral relationship emerged from laboratory school studies that used “sipping study” methods to vary the amount of methylphenidate delivered in small doses in capsules administered every 30 minutes across the day
(24,
25). These studies revealed 1) that a constant serum concentration (a “flat” pharmacokinetic profile) did not maintain full efficacy, suggesting acute tolerance to clinical doses of methylphenidate emerged each day and 2) that a rising serum concentration (an “ascending” pharmacokinetic profile) could counteract acute tolerance and maintain full efficacy for up to 12 hours.
Since 2000, several once-a-day formulations of methylphenidate and amphetamine have been developed and approved for the treatment of ADHD. These formulations are based on two processes of drug delivery: 1) an initial bolus delivery of immediate-release methylphenidate (by an overcoat or uncoated beads) designed to rapidly achieve the threshold for clinical efficacy and to produce the maximum effect within 2 hours after administration and 2) a controlled delivery of methylphenidate (by an osmotic pump process or by coated beads) designed to produce an ascending pharmacokinetic profile that would keep serum concentration above a rising threshold in order to maintain full efficacy in the face of acute tolerance
(24). Studies with methylphenidate and amphetamine indicate that serum concentrations must increase about 50% from the initial morning peak to maintain full efficacy in the afternoon (
Figure 2). On the basis of this design, the second generation of once-a-day formulations of methylphenidate and amphetamine have been rapidly accepted in clinical practice and now are prescribed in most (over 75%) cases for the treatment of ADHD in the United States.
Temporal Course of Reinforcing Effect
The speed of drug delivery to the brain affects the reinforcing effects of drugs
(26,
27). Routes of administration that produce relatively fast brain uptake—injecting, smoking, or sniffing—are more reinforcing than oral administration, which produces relatively slow brain uptake
(28).
Until recently, pharmacokinetic studies were limited to measurements in body fluids such as blood or urine. However, by using drugs labeled with carbon-11 (a positron emitter with a 20-minute half-life), which does not affect their pharmacological properties, PET imaging can be applied to directly investigate pharmacokinetic properties of [
11C]cocaine and [
11C]methylphenidate in the human brain and body
(4). In the human brain, these drugs accumulate mostly in the striatum where they bind to dopamine transporter
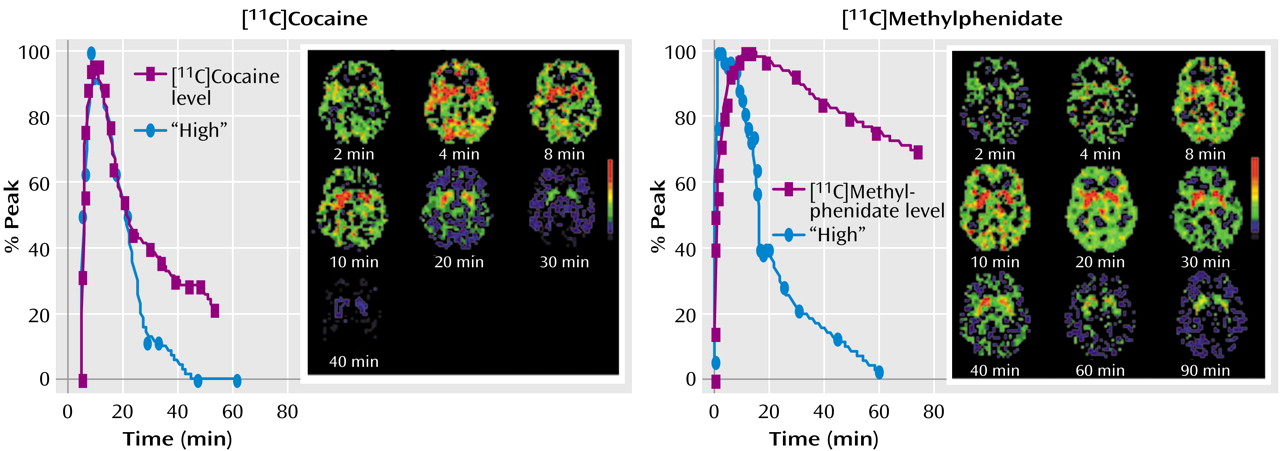
(4). Following intravenous dosing, uptake in the brain is very fast for both [
11C]cocaine (4–6 minutes) and [
11C]methylphenidate (6–10 minutes) (
Figure 3), and for both drugs, the onset of the perceived “high” parallels the fast uptake of the drugs in the striatum, with the peak for the “high” reported at about the same time as the peak striatal concentration.
In contrast to these very similar and short values of T
max, the half-life (T
1/2) for cocaine and methylphenidate differed dramatically: for [
11C]methylphenidate, T
1/2 was much longer (90 minutes) than that seen with [
11C]cocaine (20 minutes). Despite this four- to fivefold difference, the duration of the “high” was about the same for cocaine and methylphenidate (
Figure 3). For cocaine, the decline of the “high” paralleled the clearance of [
11C]cocaine in the striatum and returned to baseline when most of the [
11C]cocaine had left the brain. For methylphenidate, however, the “high” returned to baseline even while the striatal levels of [
11C]methylphenidate remained high (80% of peak). These pharmacokinetic and behavioral properties of methylphenidate derived from PET studies suggest that acute tolerance occurs to the reinforcing effects of intravenous methylphenidate, which is consistent with studies of cocaine that show that the “high” from cocaine also dissipates rapidly even when high plasma levels are maintained by repeated intravenous administration
(29) or by infusion
(30).
Thus, the “behavioral/reinforcing half life” of intravenous methylphenidate is much shorter than its pharmacokinetic half life (
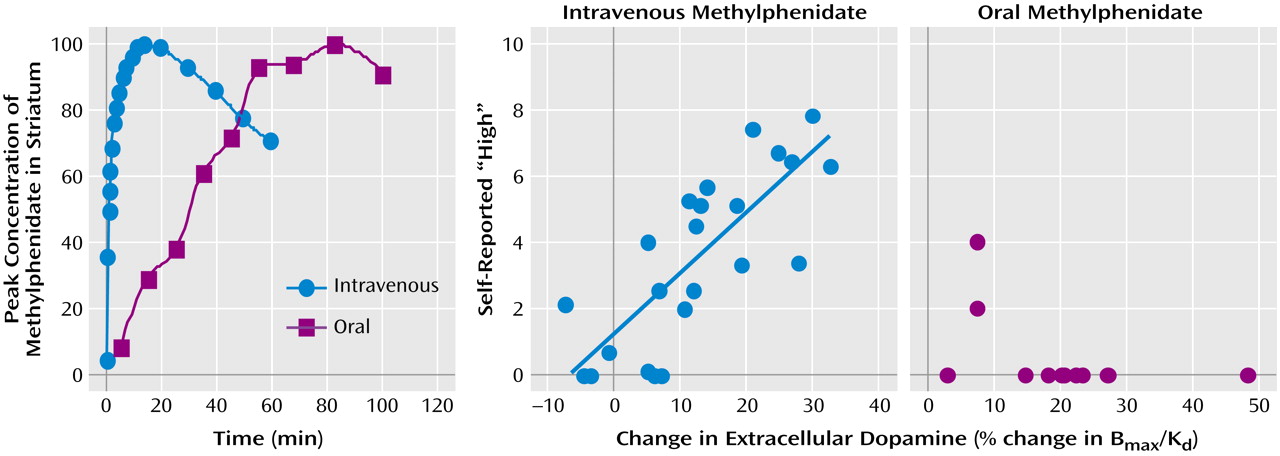
Figure 3). This dissociation suggests that the initial fast dopamine transporter blockade (and rapid increases in synaptic dopamine) is associated with the “high” and not the continuous blockade of the dopamine transporter (or consistently high levels of synaptic dopamine). Indeed, in PET studies that evaluated the relationship between methylphenidate-induced dopamine increases and their reinforcing effects, when equivalent levels of dopamine increases were established for intravenous and oral methylphenidate, intravenous methylphenidate induced a “high” but oral methylphenidate did not
(13,
31). PET measures of dopamine increases were done using [
11C]raclopride, a D
2 receptor radioligand that competes with endogenous dopamine for D
2 receptors so that its binding to the receptors is decreased when dopamine increases
(32). These studies showed that with the intravenous administration of methylphenidate (dopamine measures collected 5 minutes after its administration when peak behavioral effects were observed), but not with the oral administration (dopamine measures collected at 60 minutes when peak behavioral effects were observed), the intensity of the “high” was significantly correlated with the dopamine changes (
Figure 4). The behavioral differences between oral and intravenous administrations parallel the differences in the rate at which they reach peak brain concentrations (less than 10 minutes for intravenous methylphenidate and about 1.5 hours for oral methylphenidate), which are assumed to reflect the rate at which they increase dopamine (
Figure 4). This would suggest that the relevant variable for reinforcement is the magnitude of the dopamine changes per time unit.
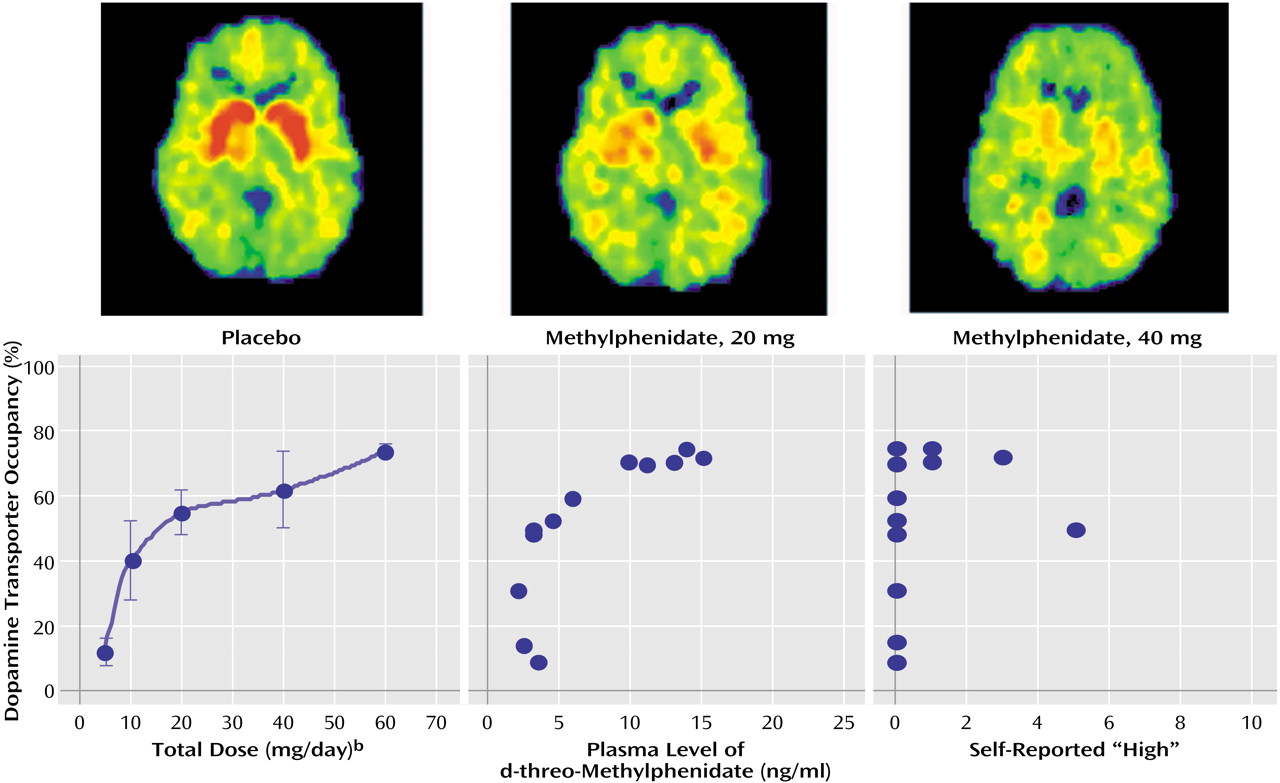
It should also be noted that even though T
max is about the same for any dose of methylphenidate, the time at which serum concentration would cross any threshold value is related to dose, with higher doses exceeding thresholds faster than lower doses. Thus, for very large oral doses, the time required to achieve a critical level for serum concentration and dopamine transporter blockade (e.g., 50%–80%) may be similar to that for low intravenous doses, which could account for why large oral doses produce reinforcing effects in some subjects
(10).
Half-Life and Clearance of the Drug From Brain
Although much less investigated, the T
1/2 and clearance of stimulant drugs from the brain is also likely to affect its reinforcing effects. If a drug blocks greater than 50% of dopamine transporter with a single administration but then has slow clearance, then dopamine transporter saturation will occur with repeated frequent administration. In the case of methylphenidate, its slow clearance may also limit self-administration because of the persistence of side effects, whose duration parallels the temporal course of methylphenidate in the brain
(33). We predict that drugs that block dopamine transporter but have very fast clearance, such as cocaine, are much more likely to promote frequent self-administration than drugs with relatively slow clearance such as methylphenidate
(4). We also predict that fluctuating versus steady-state drug concentrations in brain will affect the drug’s reinforcing effects. This prediction is based on animal studies that show that the rate at which animals self-administer stimulant drugs is associated with the downward slope of dopamine that follows the drug-induced increases in the nucleus accumbens
(34,
35). In this respect, drugs with longer half-lives or delivery systems that maintain plasma levels close to or higher than thresholds of efficacy for long time periods are less likely to be abused than drugs such as cocaine that have a very fast T
1/2.