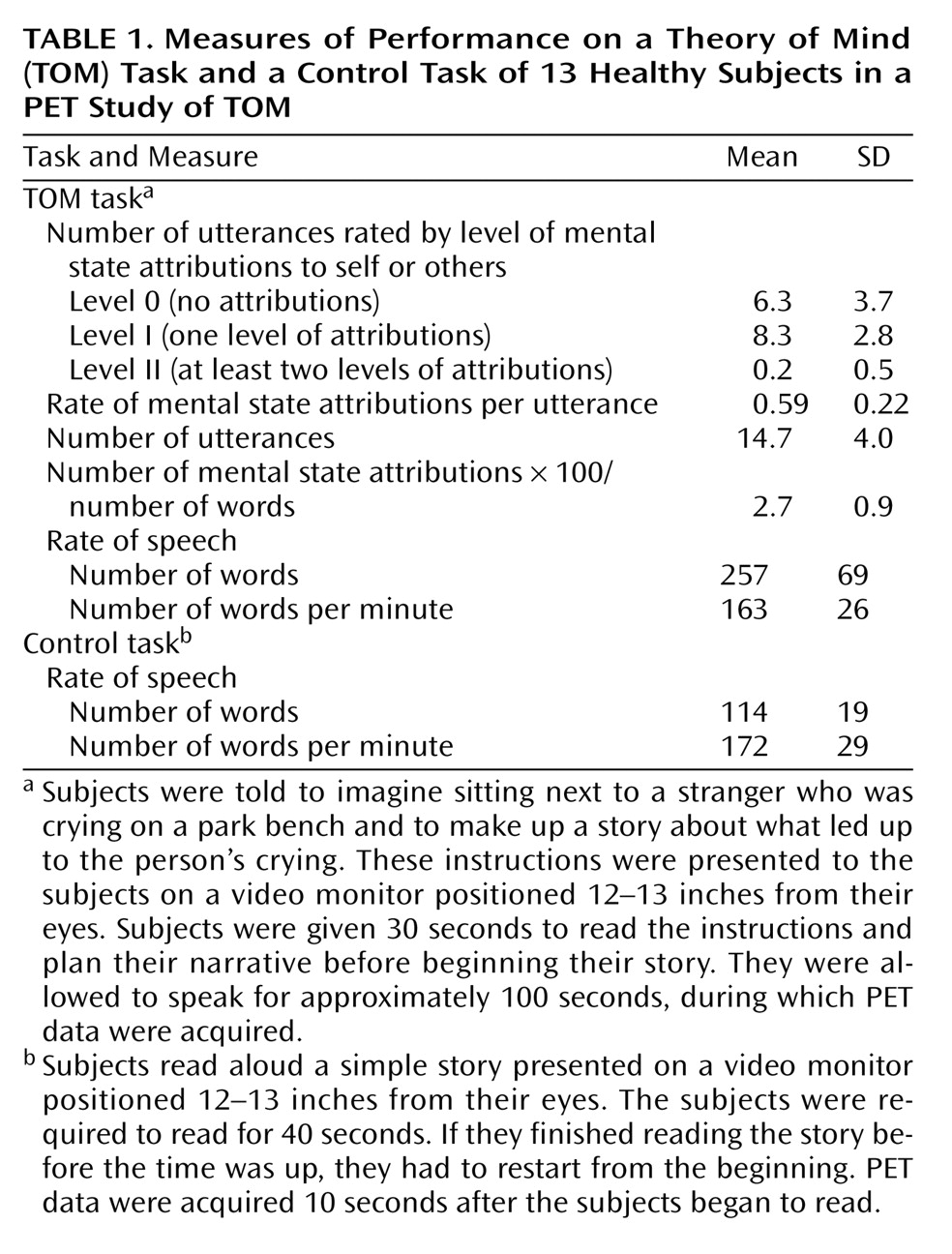
Several recent studies have begun the process of exploring the neural basis of theory of mind (TOM): how networks and faculties within our brains and minds activate and interact when we perform mental activities that require us to understand the subjective reality of other people. These studies have used a variety of imaging techniques, designs, and test materials.
In one of the first positron emission tomography (PET) studies on TOM, Goel et al.
(8) showed activation of the left medial frontal gyrus and the left middle and superior temporal gyri when they compared normal subjects making inferences about the minds of others (from Columbus’s era) to a baseline condition of making inferences about the physical world. Another early PET study compared subjects on comprehension of “TOM” stories, “physical” stories, and unlinked sentences, and it also revealed a selective activation of the left medial frontal lobe in the TOM condition
(9). A more recent PET study
(10) used a nonverbal task involving cartoons to compare attribution of intent with several control conditions, and it too found medial frontal activations, as well as others in the bilateral temporal lobes and cerebellum. In an fMRI study, Gallagher et al.
(11) showed their subjects stories and cartoons requiring both TOM and non-TOM processing. They found activations in the medial prefrontal gyrus and in the temporoparietal junctions during the TOM task. Taken together, these studies have begun to identify some of the regions involved when human beings attribute mental states to others, and in particular they suggest that medial frontal brain regions play an important role.
Therefore, we chose to conduct a PET study of TOM using a task that requires subjects to actively place themselves in another person’s place and to attribute mental states to that person. We asked them to imagine and describe the experience of a stranger met during a chance encounter on a park bench. While we measured regional cerebral blood flow (rCBF) using PET, healthy subjects verbally described what the stranger had experienced. We analyzed the subjects’ utterances in order to assess their “mentalizing” capacities. The abilities examined during this task are similar to those used by psychiatrists when they take a patient’s history and attempt to imagine the internal experience of another person. They are also similar to those used in many types of psychotherapy, including psychodynamic psychotherapy. Therefore, this study provides an opportunity to begin to understand the neural basis of the mental processes that are used in some aspects of the practice of psychiatry.
Discussion
This study adds to the growing literature on TOM by examining a complex mentalizing task that requires the use of language and active imagining of another person’s mental state. It replicates some earlier findings and adds some new ones. The TOM task investigated in this study activated a distributed group of brain regions that are engaged when individuals attempt to imagine and verbally describe the subjective state of another individual whom they have never met before. It is related to the state that psychiatrists think of as “the empathic mode.”
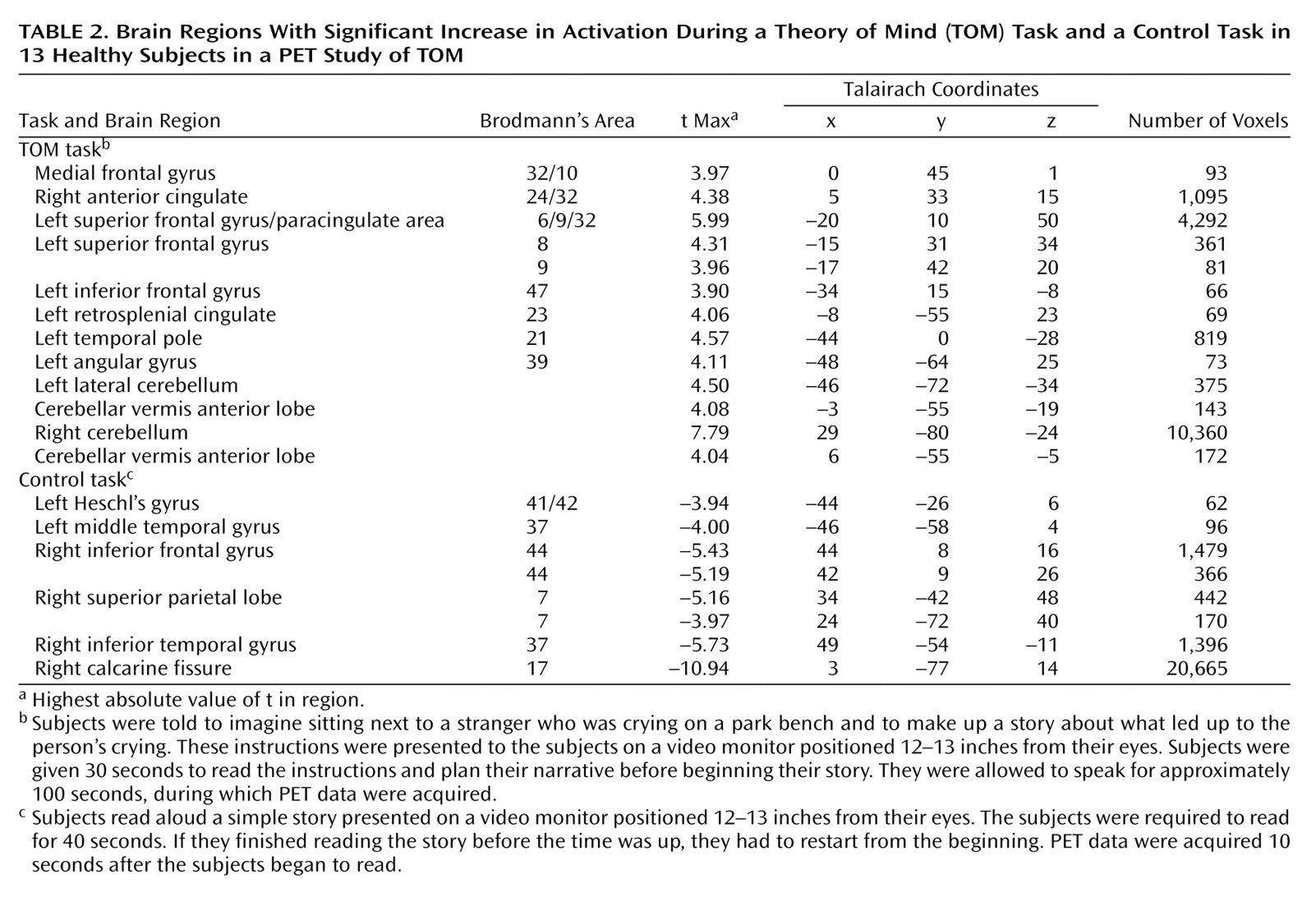
The medial frontal cortex appears to be a crucial region for this TOM task. Like the four previous studies that have used PET or fMRI to investigate TOM
(8–
11), this study showed that the medial frontal region is activated when human beings attribute mental states (
Figure 1). The function of this region is thought to be maintenance of a representation of the mental state of self
(12). The previous four studies that also found medial frontal activation were all quite different in their designs and stimulus materials: pictures of various kinds of objects for which subjects must infer the objects’ normal use and model another person’s knowledge about the objects’ use
(8), inferential versus factual stories
(9), cartoons versus stories that also contrast facts with jokes and deception
(11), and cartoons that contrast intent with physical causality
(10). Yet these different designs found medial frontal activation, predominately in the left hemisphere, during the TOM condition. Therefore, we can conclude that this region must be centrally involved in the core process of mentalizing in a manner that is independent of the modality through which the TOM task is presented (e.g., visual versus verbal).
This study differs from its predecessors in two significant respects that may affect its results and illuminate other components of TOM. Unlike the previous studies, which required passive viewing of stimuli and some type of simple response (often pushing a button in response to a question), this study asked subjects to actively imagine the internal state of another person and to describe that internal state in extended verbal discourse. This task required using both language and memory to create a TOM discourse. As shown in the behavioral data, subjects made an attribution of mental states in 59% of their utterances, indicating that this task was appropriate for tapping into TOM. There was no difference in the rates of words produced per minute between the experimental and control tasks. Therefore, we can conclude that the verbalization component of the TOM task was subtracted out through the control task. Furthermore, the classic “language production regions” (e.g., Broca’s area, Wernicke’s area, sensorimotor speech areas) were not seen as activated in the experimental task, adding to the evidence that this task successfully examined TOM.
Since making up a story about another person does require the creative and spontaneous use of language (which the control task did not), however, we cannot assume that language components were completely eliminated. Nor is such elimination necessarily desirable, given the extensive literature suggesting that language is a crucial component of TOM
(10,
17–
19,
38). Likewise, it has been suggested that TOM recruits episodic memory (recollections of past personal experience)
(16–
21). TOM is a complex cognitive process, which is likely to require that subjects mentally reference their own past experiences as they attempt to imagine what the other person has experienced. Therefore, the distributed activations observed in this study must be interpreted in the light of the possible participation of these additional brain systems, which must to some extent participate in the “TOM system” when it is challenged by a complex empathic task.
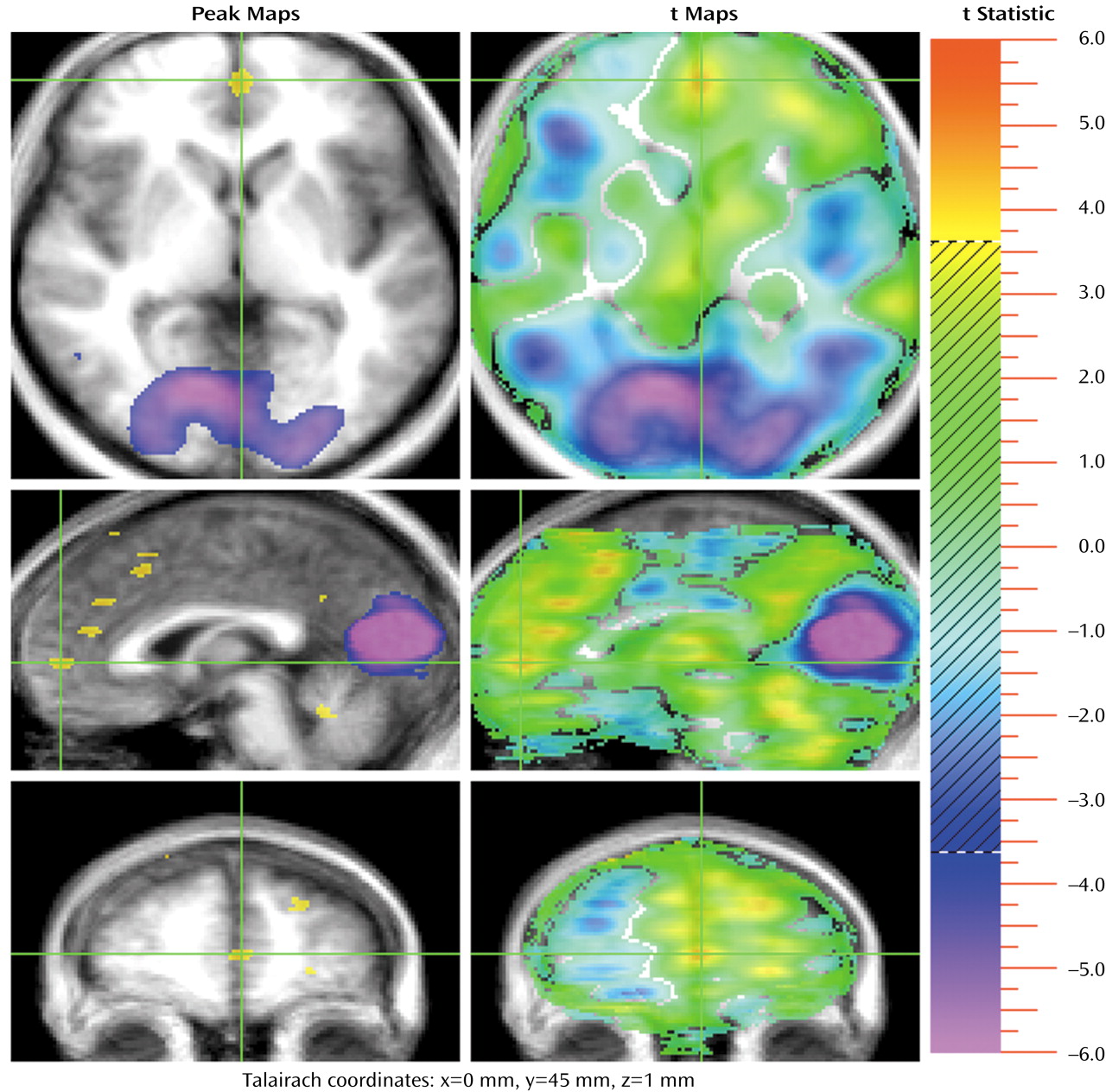
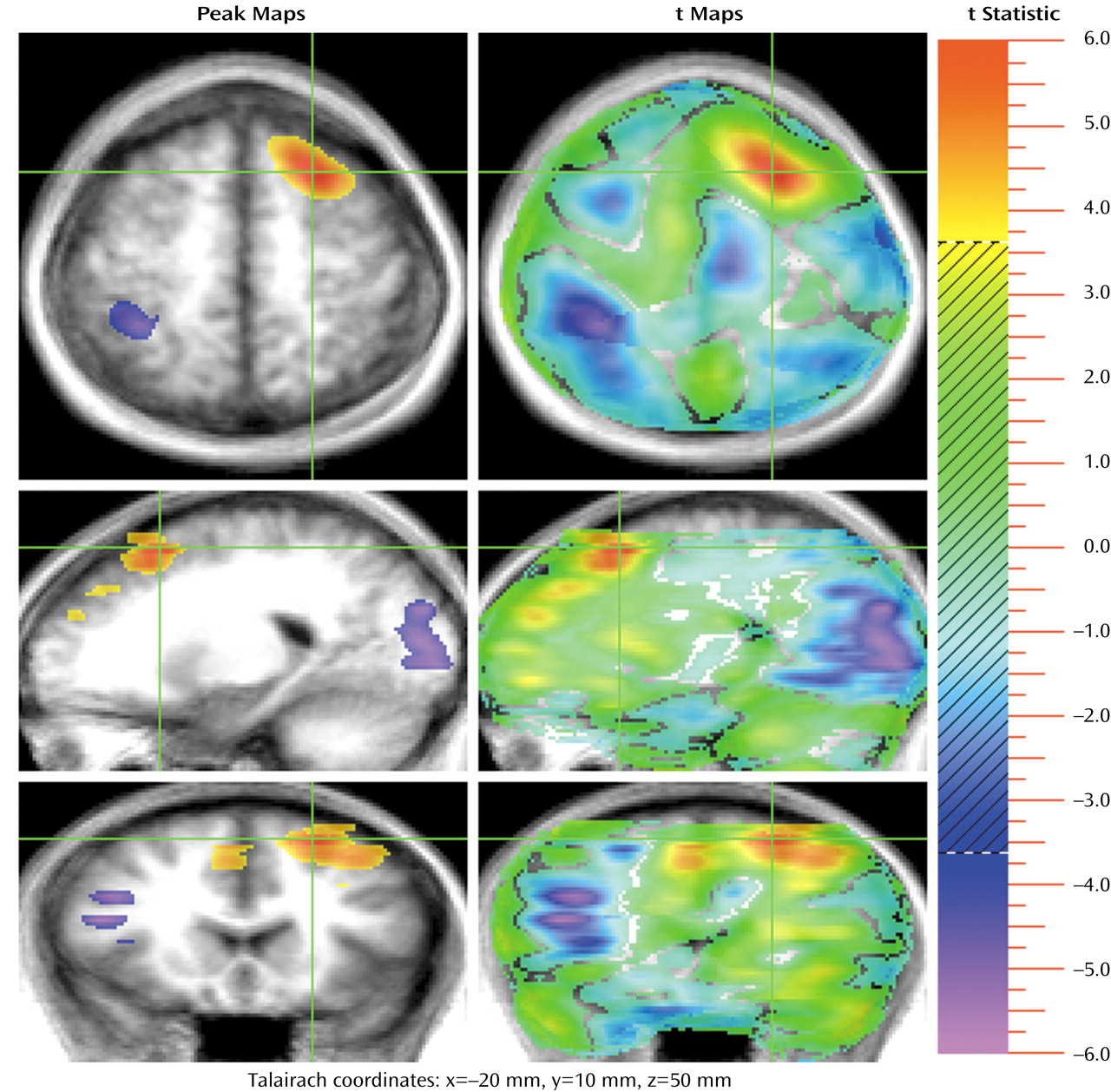
The medial frontal activations observed in this study are part of a large cluster of frontal activations. One group occurs in the superior frontal regions, with three significant activations that include a more anterior activation (Talairach coordinates: x=–17, y=42, z=20), a more rostral one (coordinates: x=–15, y=31, z=34), and finally the most posterior one (coordinates: x=–20, y=10, z=50) (
Figure 2). The most posterior activation is very large (4,292 voxels) and divides into two separate activations if a higher significance threshold is used. At a threshold of 4.2, this activation separates into two: one centered in the superior frontal gyrus (coordinates: x=–20, y=9, z=50; 4.4 cc; t max=5.99) and one in the anterior cingulate (Brodmann’s area 32) (coordinates: x=–5, y=14, z=40; 0.5 cc; t max=4.76). The function of these multiple superior frontal regions is not definitively known, but it is likely that they reflect various aspects of medial frontal functions. That is, they may contribute to the emotional, cognitive, and action components of representation of the self
(12).
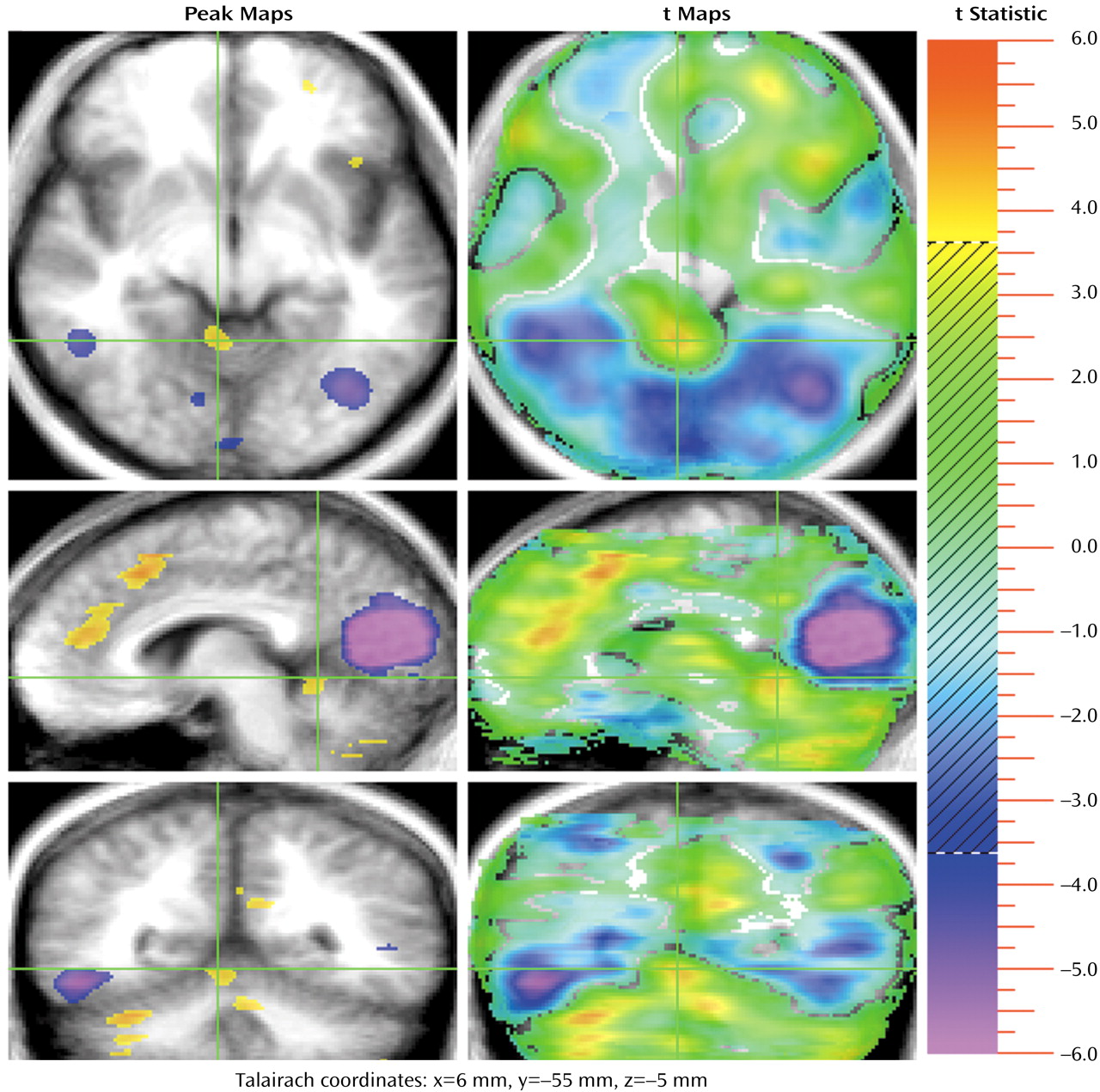
In addition, a second group of frontal activations occurs in several regions of the cingulate gyrus (
Figure 1 and
Figure 4). The activations are bilateral, but the largest (coordinates: x=5, y=32, z=15; 1,095 voxels) is on the right (with some extension into the left hemisphere). The cingulate gyrus has been extensively studied in functional imaging studies, as well as in lesion and animal studies. A recent review proposed that the role of the anterior cingulate is to juxtapose emotions with focused problem solving, error recognition, self-control, and adaptive response to changing conditions
(39). The cingulate gyrus is widely connected with diverse parts of the brain and appears to be central for coordinating and focusing attention on complex tasks. Several lesion, animal, and electrophysiological studies have identified an anterior “emotional” part of the anterior cingulate and another more posterior “cognitive” region
(40,
41). Thus, the multiple cingulate activations appear to reflect efforts to integrate the emotional and cognitive components of the TOM task.
A retrosplenial cingulate activation is also present. This region has been shown in several imaging studies to play a role in the expression of emotions, as well as in episodic memory
(42–
44). Working in conjunction with the other activated regions, the retrosplenial cortex may be used for focusing attention on an emotionally charged topic, retrieving personal experiences that will assist the empathic response, and attributing the retrieved information to self and others
(4,
16,
20,
21). An additional group of activations may also reflect the language and memory retrieval components of this particular TOM task. They include the activations in the angular gyrus and the anterior temporal pole (
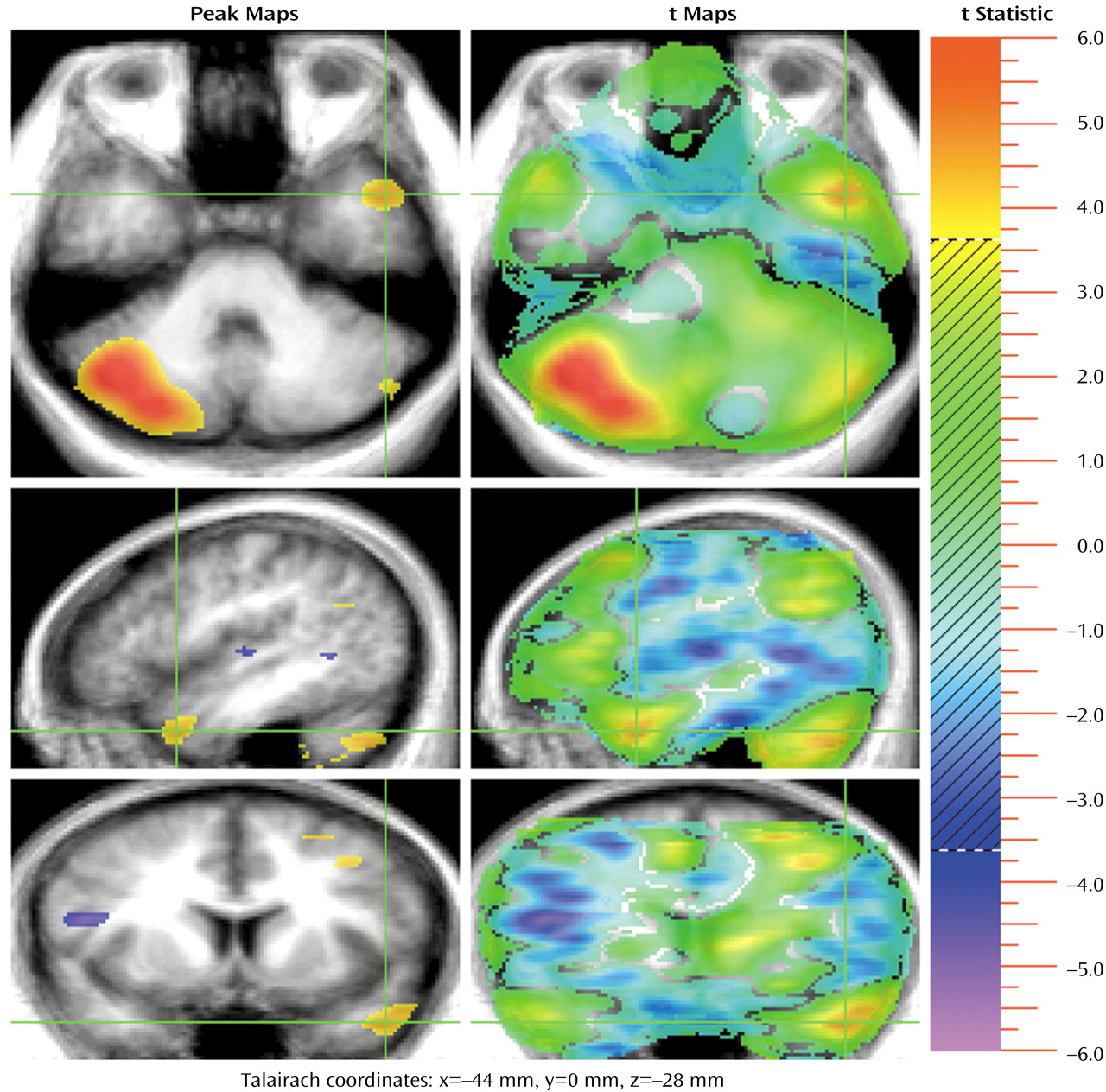
Figure 3). The angular gyrus is a parietal association region that has connections to the anterior temporal pole, as well as to prefrontal regions
(19). These regions may be activated by the search for specific words, for story content, and for episodic memories. Both frontal and anterior temporal regions have been shown to be active in verbal memory tasks that require verbal recall of word lists, complex narratives, and episodic memory
(42,
45,
46). Anterior temporal activations were also observed in all previous PET and fMRI TOM studies
(8–
11), suggesting that these regions may be a modality-independent part of the TOM system.
The largest activation during the TOM task occurs in the right cerebellum (10,360 voxels). This peak fills the lateral and medial aspects of the right cerebellum. There is a small mirror activation in the left lateral cerebellum. In addition, two activations occur in the anterior lobe of the cerebellar vermis (
Figure 3 and
Figure 4). These findings add to the rapidly growing evidence that the cerebellum is an important “cognitive organ” in the human brain. They indicate that the cerebellum plays a role in yet another complex “higher” nonmotor mental activity: performing a task that requires a theory of mind. Previous studies have shown that the cerebellum is activated in many other cognitive tasks such as memory for faces, verbal memory, episodic memory, error detection, attention, sensory activation and discrimination, and timing
(47–
60). Cerebellar activations were also seen in two earlier TOM studies
(10,
11). The large right cerebellar activation reflects cross-hemispheric diaschisis: the multiple left hemisphere frontal, temporal, and parietal regions used in the TOM task are linked to the contralateral cerebellum, reflecting recognized anatomic pathways
(47–
58). The two activations in the vermis may be presumed to reflect a similar link to the midline “paralimbic” activations in the cingulate gyrus. Taken together, these large and multiple cerebellar activations indicate that the cerebellum is working in concert with the cerebral cortex to coordinate the process of imagining and describing the internal state of another person, interactively finding, checking, and monitoring the creation of the TOM story. It is of interest that a growing body of literature has suggested an abnormal cerebellar structure in autism, at both the cellular and anatomical levels
(61–
64). Impaired TOM, in fact, seems to be a hallmark of this neurodevelopmental illness
(23).
Frith and his team
(12) have made extensive contributions to the TOM literature, both experimentally and theoretically. They suggested that the brain may possess a “theory of mind system,” just as it possesses other systems that have become increasingly well-mapped, such as the language system, the facial recognition system, or the object recognition system. On the basis of their experimental work and studies of brain activity in higher primates, they proposed that the TOM system is composed of three major nodes: medial prefrontal, superior temporal sulcus, and inferior frontal. They proposed that the medial prefrontal node maintains representations of the mental state of the self, that the superior temporal sulcus detects the behavior of agents and analyzes the goals and outcomes of this behavior, and that the inferior frontal region maintains representations of actions and goals
(12).
The present study, especially when examined in the context of other recent studies, adds another perspective to the nature of the TOM system in the human brain. Instead of attempting to eliminate the contributions of episodic memory and language, it includes them in the design, based on the recognition that most efforts to attribute mental states to other people depend heavily on these forms of mental activity. Two competing mechanisms have been proposed for our ability to mentalize. The simulation theory suggests that we draw from our repertoire of past experiences when we attempt to imagine the experiences of others, while the theory theory suggests that we formulate an abstract representation or set of rules (theory) and then apply that model/theory to recreate or imagine the experience of others
(2,
26,
65,
66). Both of these mechanisms inevitably must conduct the simulation or formulate the theory using language, and both must also draw on personal past experience (episodic memory). Therefore, our approach suggests that both language and episodic memory should be considered as components of the TOM system.
The TOM network may consist of a core system and “supportive” components. Lesion studies, for example, have shown some brain areas to be essential for TOM processing
(2,
67,
68). Medial frontal lesions, particularly right ventral lesions, impaired detection of deception in the study by Stuss et al.
(2), for example, suggesting that this brain area is part of the core system. As for language, there is some evidence that developing normal language skills is also important for the acquisition of TOM
(20). Impaired grammar in adulthood, however, does not seem to affect performance on mentalizing tasks
(38). This finding suggests that although language centers might contribute significantly to normal TOM processing, some components (e.g., the phonological loop) might not be as essential, while others (e.g., semantic components) may be. In this study we have identified a TOM system that is widely distributed and that is made up of interactive nodes in frontal and anterior cingulate regions, associative and memory regions in the angular gyrus and anterior temporal lobe, and the cerebellum. Many of these regions have also been found to be active during mentalizing in other TOM imaging studies.
Regardless of how the TOM system is conceptualized, the replication of so many findings across multiple studies suggests that the tools of functional imaging can indeed be used to study complex mental activities. Using the mind to create scientific studies of how one human mind can understand another has become a realizable goal. Freud’s project for a scientific psychology is now well under way.