We set out to examine whether maternal smoking during pregnancy is associated with offspring ADHD symptoms by using a twin study design that allows us to establish the contribution of genetic influences on ADHD. We also used maternal reports of smoking during pregnancy and teacher reports of ADHD symptoms to avoid the problem of shared rater effects. We further tested whether the association remained after removing the effects on ADHD of conduct disorder symptoms, birth weight, and family and social adversity.
Method
Families of all twins, 5–16 years of age, identified from the population-based Greater Manchester Twin Register were sent a package of questionnaires
(11). Mothers were asked to complete a validated twin similarity questionnaire
(12), the Rutter A scale
(13), and the Family Environment Scale
(14), and information was requested on the number of cigarettes smoked during pregnancy (0, <10, 10–20, >20 cigarettes per day) and birth weight of each twin
(11). Data were obtained for 2,054 twin pairs (73% response rate).
Informed written consent was obtained from the parents to contact schools. Teachers were also asked to complete an ADHD rating scale (
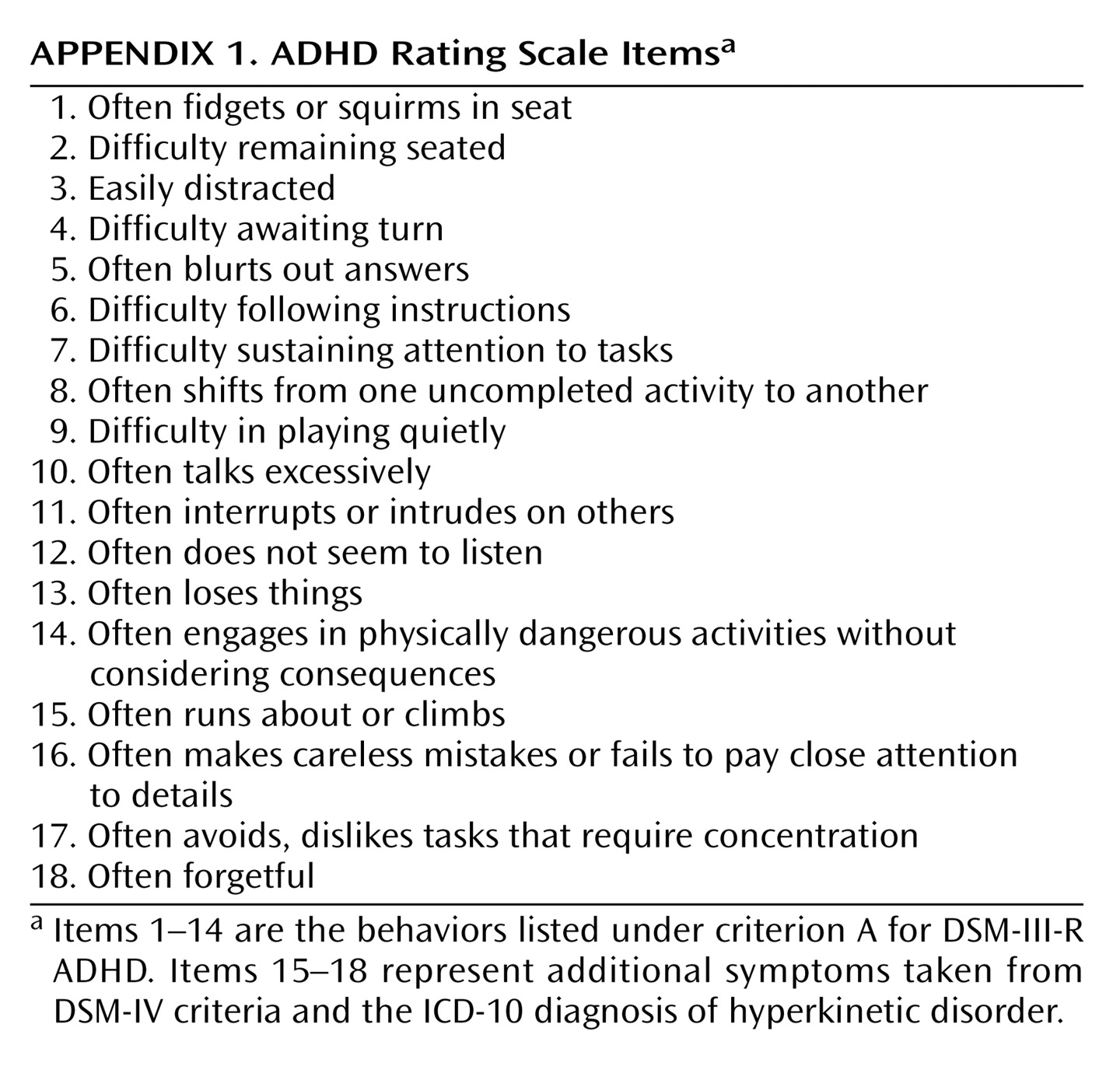
Appendix 1) on each twin
(11). This scale included the 14 DSM-III-R symptoms of ADHD with another four items added to cover additional DSM-IV ADHD symptoms and symptoms of ICD-10 hyperkinetic disorder. Each symptom was rated on a 4-point scale of severity, and each item was summed to generate a total symptom score. Teacher reports were available for a subsample of 1,452 pairs. The characteristics of the sample and responders have been described in detail previously and been shown to be representative of the local population
(11).
Data were analyzed by structural equation modeling with the program Mx
(15). The basic twin study design allows for partitioning phenotypic variance into additive genetic factors (a
2), shared/common environmental influences (c
2), and nonshared environmental influences (e
2), where c
2 is environmental variance (unmeasured) that contributes to twins in a pair being more similar, and e
2 is environmental variance (unmeasured) that accounts for twin differences (including measurement error). A heritability estimate (h
2) can be obtained by dividing the genetic variation by the total variation (i.e., a
2, c
2, and e
2 combined).
We tested several models. The first model estimated the contribution of additive genetic factors (A), shared/common environment influences (C), and nonshared environment influences (E) on teacher-rated ADHD symptoms (ACE model). This model was modified to allow for gender-specific genetic and environmental influences (gender-specific ACE model). Next, we included a measured variable, maternal smoking during pregnancy (S), to estimate the additional influence of this variable on the twins’ ADHD scores (full ACES model). The fit of this model can be compared to the one without S to establish the significance of smoking during pregnancy. The overall goodness of fit of all models is given by a chi-square value for which a smaller value indicates a better fit. The fit of the ACE model and the gender-specific ACE model were compared against the fit of the full ACES model by examining the difference in chi-square goodness of fit values. We also present Akaike’s information criterion (AIC) for each model. This criterion provides a measure of goodness of fit and parsimony with the most satisfactory fit shown by the lowest, most negative value
(16). ADHD symptom scores were log transformed to approximate normality by using the transformation ln (1+x) as well as covariance matrices generated before undertaking model fitting.
Finally, given that smoking during pregnancy covaries with other factors, models were rerun after removing variance in ADHD scores attributable to social adversity (family size, social class, family conflict), birth weight, and antisocial symptom scores.
Results
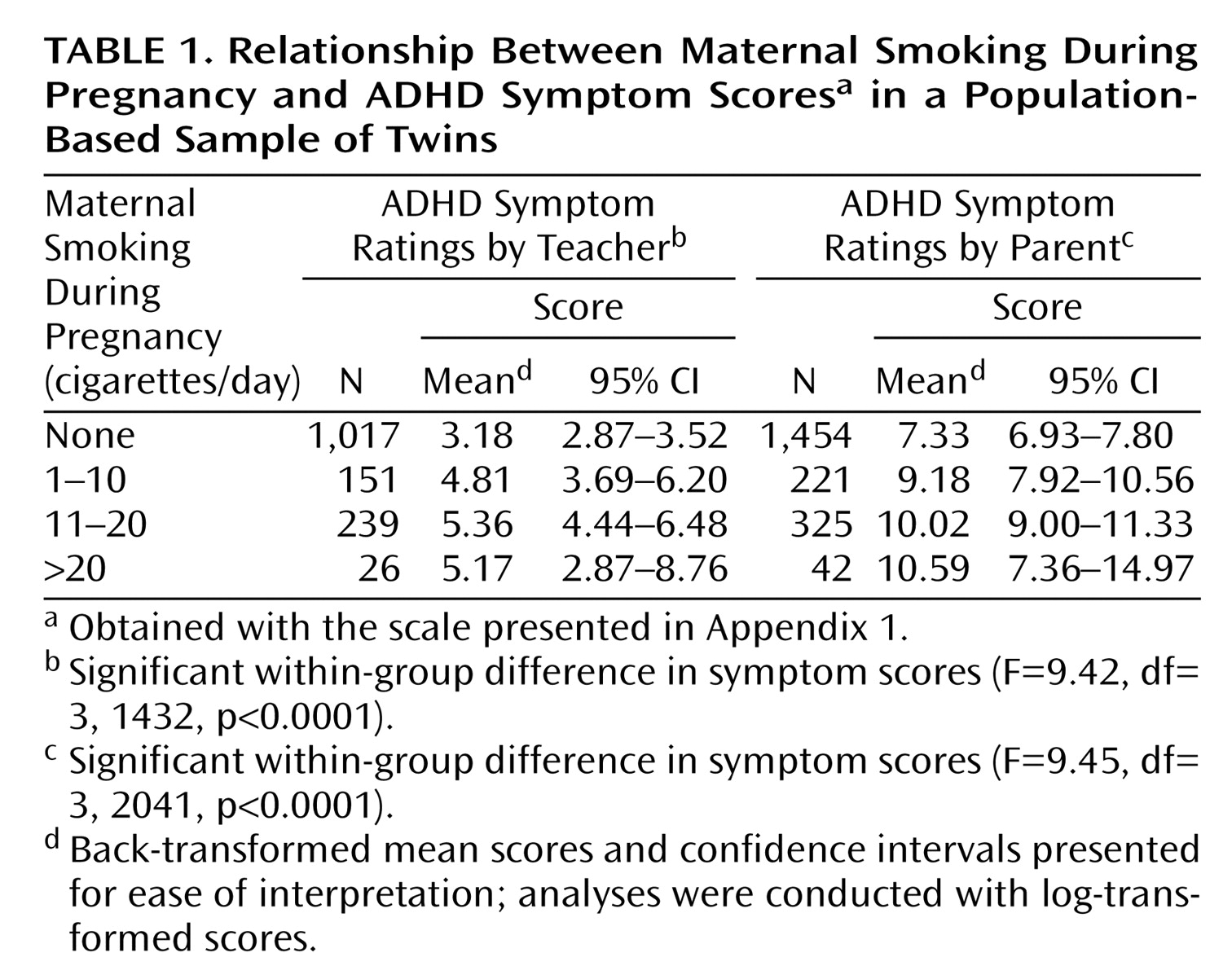
Table 1 shows mean offspring ADHD symptom scores (using scores of one twin from a pair) according to the number of cigarettes the mother smoked during pregnancy. Both parent and teacher ratings of ADHD symptoms are presented to show that there was a strong association between maternal smoking during pregnancy and offspring ADHD symptom scores regardless of rater.
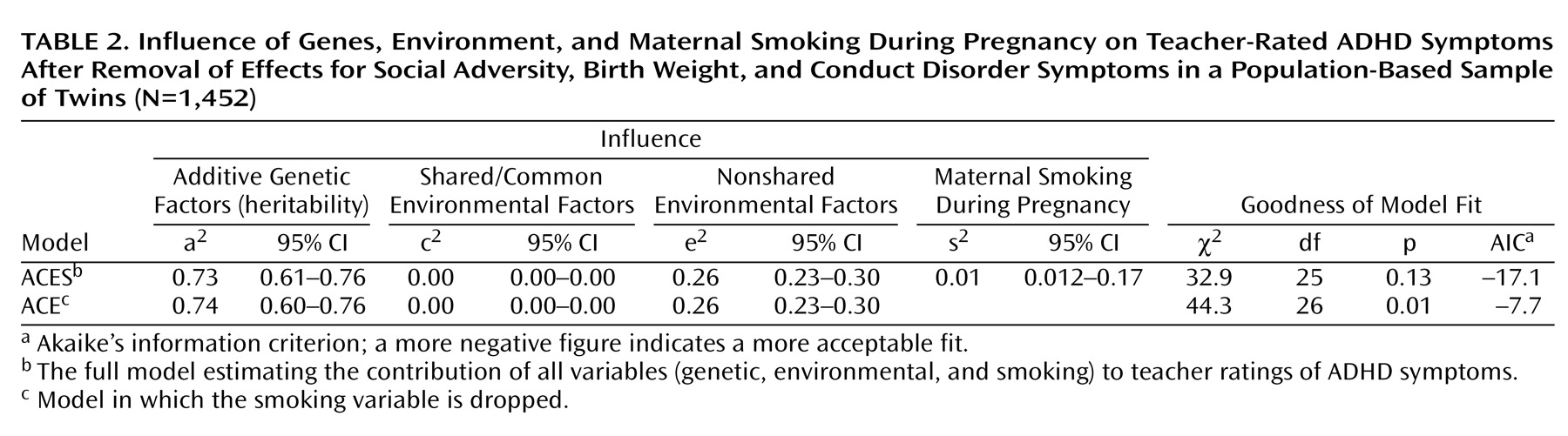
We first fitted models to unadjusted ADHD scores. The full model incorporating all variables (genetic, environmental, and maternal smoking) provided a reasonable fit to the data (ACES model: χ2=32.5, df=22, p=0.07; AIC=–11.5). The parameter estimates in this model could be equated for boys and girls without a significant worsening of fit (χ2=36.2, df=25, p=0.07; AIC=–13.79), indicating that there were no significant gender effects. However, smoking effects could not be dropped without a significant deterioration in fit (ACE model: χ2=74.7, df=26; AIC=22.7). The difference between the model with and without the maternal smoking variable was χ2=38.5, df=1, p<0.001, indicating that the effect of smoking during pregnancy is highly significant. Thus, our model indicates that genetic factors (a2=0.67), shared environmental influences (c2=0.08), nonshared environmental influences (e2=0.23), and maternal smoking during pregnancy (s2=0.02) influence teacher-rated ADHD symptom scores.
Finally, we reran the models after removing the effects of social adversity, birth weight, and antisocial symptom scores. These results are shown in
Table 2. Although the shared environmental influences (8% in the original full model with unadjusted ADHD scores) disappeared once these factors had been taken into account, the influence of smoking during pregnancy remained significant, and this variable could not be dropped without a significant deterioration in fit (χ
2=11.4, df=1, p<0.001).
Discussion
We found that maternal smoking during pregnancy shows a significant association with offspring ADHD symptoms that is additional to the influence of additive genetic factors and nonshared environmental influences. This finding adds to the increasing literature on prenatal smoking and ADHD/behavioral problems. To our knowledge, this is the first population-based study to show an association between maternal smoking during pregnancy and ADHD symptoms.
We found that smoking during pregnancy accounted for only a small proportion of the total variance in ADHD symptom scores. Therefore, it can be questioned whether the finding is of importance. ADHD is considered to be a complex disorder that is probably influenced by a variety of different genetic and environmental factors. When the effects of a single variable, whether genetic or environmental, on a complex disease are considered, we can expect to find that only a small proportion of phenotypic variance is accounted for by that single variable. There is evidence that the risk effects of environmental factors for child psychopathology arise from the action of multiple factors rather than a single variable
(17). Similarly, in molecular genetic studies aimed at identifying specific susceptibility genes, the evidence so far suggests individual genetic variants associated with ADHD
(10) (and other disorders such as schizophrenia), when considered alone, also account for a very small increase in risk
(18). In twin studies such as this, we can thus expect that the contribution of a single measured environmental risk factor or genetic variant to phenotypic variance is likely to be small. In contrast, estimates of latent genetic (heritability) and environmental variance (c
2/e
2) will appear much larger, since they are likely to include the effect of many unmeasured genetic and environmental factors, although it will be unknown which specific genes and environmental factors these are.
There have been numerous studies showing that ADHD is genetically influenced, with reported heritability estimates from twin studies of 60%–91% according to parent reports and 39%–72% according to teacher reports
(19). Has there been previous evidence of additional environmental influences on ADHD such as maternal smoking during pregnancy? First, genetic studies have shown that environmental factors also contribute to ADHD. However, these studies, including our own previous work, have statistically inferred the contribution of environment. To our knowledge
(19), only one of more than 14 twin studies
(20) and no adoption studies of ADHD have included measured aspects of the environment. No twin studies to date have specifically included the effects of maternal smoking on ADHD. This is important, given that including direct, rather than latent, measures of environmental factors increases the statistical power to detect environmental effects
(21).
Second, there is evidence that many “environmental” risk factors are not independent of genetic influences, with twin studies showing that variables such as life events and parenting are to some extent heritable
(22–
24). Smoking is an example of such a risk factor, given that it is known that smoking behavior is genetically influenced. In twin studies, phenotypic variance (in the offspring) that is attributable to environmental factors that are influenced by genotype (and maternal smoking during pregnancy is likely to be such a factor) will be subsumed within the heritability estimate, even when there is a truly environmentally mediated risk effect
(4). Thus, the association of prenatal smoking with ADHD symptoms in our study will almost certainly have been underestimated. Much of the effects of prenatal smoking are likely to be included in our heritability estimate. That is, if smoking during pregnancy is genetically influenced and the association with ADHD in offspring is genetically mediated, then those effects will contribute to greater monozygotic twin similarity than dizygotic twin similarity (i.e., contribute to the heritability estimate).
Moreover, the twin study method is based on the equal environments assumption, whereby monozygotic twins are assumed to share environment to the same extent as dizygotic twins. If monozygotic twins share a more similar intrauterine environment than dizygotic twins in terms of exposure to the products of cigarettes, then again these effects would be subsumed within the heritability estimate. We are unable to separate out these effects with our study design and have focused simply on testing whether or not we can detect effects of maternal smoking during pregnancy on ADHD that are additional to those effects that may be subsumed in the heritability estimate
(9).
As mentioned earlier, many but not all of the previous studies have focused on antisocial behavior or conduct disorder
(4,
25). Thus, it has not been entirely clear whether the association between smoking during pregnancy and ADHD is attributable to comorbidity with conduct disorder. Our findings concur with those of a recent case-control study of ADHD in a clinical sample
(1) in showing that the effect of maternal smoking during pregnancy on ADHD symptoms remained significant when conduct disorder symptoms were controlled. Most of the available evidence suggests that smoking during pregnancy is associated with early-onset, life course-persistent antisocial behavior
(6,
8), which is known to be preceded by earlier hyperactivity
(17). Thus, it is possible that the previously observed association of prenatal smoking with antisocial behavior may be mediated through ADHD. Clearly, this needs to be tested by using a longitudinal design.
In keeping with previous studies of maternal smoking during pregnancy and ADHD
(1,
2), we also find no evidence of gender differences. Finally, if mothers rate a child’s symptoms, there is the risk that the observed association with smoking during pregnancy may be influenced by rater effects. We overcame this difficulty of informant-specific effects by using teacher rather than parent ratings of ADHD.
Clearly, despite findings of an association between maternal smoking during pregnancy and offspring ADHD symptoms from this population-based study as well as a previous clinical study, much caution is needed before drawing conclusions that there is a causal relationship. Nevertheless, there has been increasing interest in the potentially neurotoxic effects on offspring of smoking during pregnancy. Animal studies, although not entirely consistent, have suggested an association between in utero exposure to cigarette smoke and increased locomotor activity and learning deficits
(4). There is also some evidence to suggest that prenatal exposure to nicotine may affect neural development as well as neurotransmitter systems. Thus, although at present it is impossible to draw conclusions as to whether the association between maternal smoking during pregnancy and offspring ADHD symptoms is to some extent causal, there is sufficient work on potential biological mechanisms to warrant that this be further examined.
There are several limitations to this study. First, similar to previous epidemiological work
(1), every potential confound has not been measured. We had no data on parental psychopathology or drug and alcohol use. Although all the genetic effects of unmeasured variables would have been included in our model, we cannot rule out the possibility that some other unmeasured environmental variable that is associated with smoking during pregnancy accounted for our findings. Nevertheless, other studies that have included parent psychopathology (including ADHD) and drug and alcohol use (but not genetic influences) have found an independent association between smoking during pregnancy and ADHD
(5).
Second, the analyses are based on questionnaire-derived ADHD symptoms rather than clinical disorder. Nevertheless, there is evidence to suggest that ADHD appears to behave as a continuum in terms of etiology
(10). However, as dimensional measures provide greater statistical power to detect significant effects, we may have failed to show an association of maternal smoking during pregnancy with a binary measure of clinical ADHD.
Third, given the sample size and the type of models being tested, it could be argued that it would not be possible to drop any measured environmental variable. This did not, however, seem to be the case. When we checked for this by running models incorporating another environmental variable (family conflict), the item could be dropped without deterioration in fit. Fourth, smoking data were obtained retrospectively from maternal reports, and thus we cannot rule out the possibility of recall bias.
Fifth, although the response rate was 91% for teachers who were approached, teacher response rate for the total twin register was 1,452/2,814 (52% of the total sample). The pattern of monozygotic and dizygotic twin correlations for maternally rated ADHD symptoms (and the association with maternal smoking during pregnancy) in twins whose parents who did not give consent to contact schools was similar to those with parents who allowed us to approach schools. However, we cannot rule out the possibility that teacher-rated responses would have differed in the two groups.
Finally, even twin or adoption designs cannot be used to test whether or not maternal smoking during pregnancy has a truly causal relationship with offspring ADHD symptoms, independent of genetic factors, not even where maternal ADHD is assessed. Strictly speaking, teasing apart genetic and environmentally mediated effects would require examining offspring exposed to maternal smoking in utero where the intrauterine environment was provided by a nongenetically related “mother” (surrogate; that is a “before-birth” adoption study design). That is clearly not a feasible design in humans. Thus, we are careful in stating that we observe an association between maternal smoking during pregnancy and offspring ADHD symptoms and do not conclude that this necessarily implies causality.
In conclusion, it is striking that clinical and now twin study findings have been remarkably consistent in showing an association between maternal smoking during pregnancy and offspring ADHD symptoms
(4). Our findings extend previous work by being the first to demonstrate that the association of prenatal smoking with ADHD remains even when the additional substantial genetic contribution to ADHD symptoms is included and when we examine association between data from different raters in a population-based sample.