Evidence for deleterious effects of alcohol intoxication and withdrawal on both the developing and mature brain is extensive (reviewed in reference
1). Both cross-sectional
(2) and longitudinal
(3) imaging research has suggested greater brain shrinkage during adulthood in subjects with alcohol dependence relative to comparison subjects. The precise relationship between alcohol exposure and brain volumes within alcohol-dependent subjects, however, has not been well characterized. In this report, we use volumetric magnetic resonance imaging (MRI) to determine if, within alcohol-dependent subjects, individual differences in brain atrophy relate to specific parameters of drinking history. In addition, many alcohol-dependent subjects also have comorbid marijuana or cocaine use disorders, and the effects of chronic collateral substance use on brain morphometry in subjects with alcohol dependence are not well understood.
Cocaine use disorder in subjects with alcohol dependence is of particular interest for several reasons. First, cocaine abuse may damage the brain at a level that could be detected morphometrically. Cerebral vasospasm and impaired blood perfusion (ischemic events) induced by cocaine and its metabolites have been implicated in cocaine-mediated neurotoxicity
(4). In addition to an elevated incidence of apparent stroke in cocaine-abusing patients (e.g., reference
5), MRI research has also revealed an elevated incidence of subclinical vasculotoxic lesions in cocaine-dependent subjects relative to comparison subjects, especially in older subjects
(6). Acute cocaine challenge dose-dependently increased the likelihood of vasoconstriction as detected by magnetic resonance angiograms
(7), where the likelihood of vasoconstriction was positively correlated with extent of previous cocaine use.
Second, the likelihood of cocaine-mediated neurotoxic vascular events may be especially high in alcohol-dependent subjects, since laboratory studies have indicated that ethanol administered in tandem with large, cumulative doses of cocaine elevates the heart rate to a degree greater than the sum of single-drug effects. This interaction was further magnified when subjects were engaged in a task and not at rest (reviewed in reference
8). Cocaethylene, a long-lasting vasoactive metabolite resulting from cocaine and ethanol coadministration, has been implicated in both vasoactive/neurotoxic
(9) and reinforcing
(10–
12) effects of coadministration of cocaine and ethanol. Thus, alcohol-dependent subjects may be especially prone to adverse neurovascular events following cocaine administration.
Third, focusing on alcohol-dependent subjects can help isolate cocaine effects. Namely, some of the brain morphometric characteristics of cocaine abusers, such as 1) reductions in gray matter relative to comparison subjects in several regions
(13), 2) ventricular enlargement
(14), 3) blunted age-related increases in frontal and temporal white matter
(15), and 4) accelerated age-related reductions in temporal lobe gray matter
(16), are not only similar in many respects to characteristics seen in subjects with alcohol dependence but may have been confounded by heavy alcohol consumption in persons with diagnoses of cocaine abuse or dependence
(17). For example, a large-scale community survey revealed that while only 20% of male respondents 18–35 years of age who endorsed alcohol use in the past year also endorsed cocaine use, nearly 100% of 18–35-year-old male respondents who endorsed cocaine use in the previous month also endorsed alcohol use, frequently (85%) in conjunction with cocaine
(18). To our knowledge, no morphometric analysis has concurrently assessed the independent effects of alcohol versus cocaine use. One means of parsing out specific cocaine effects on brain atrophy then is by reducing intersubject variance in heavy alcohol use by specifically assessing whether among alcohol-dependent subjects, those with a comorbid cocaine use disorder show especially pronounced brain atrophy.
Finally, demonstrating a dose-dependent relationship between alcohol exposure and brain volume measures
within alcohol-dependent subjects avoids some confounds found in comparisons between alcohol-dependent subjects as a group and healthy comparison subjects. Namely, (premorbid) depressive symptoms
(19) and even personality measures of neuroticism in nonpsychiatric comparison subjects
(20) have negatively correlated with brain volumes. Given the pervasiveness of depression and anxiety within alcoholism, it is unclear how much of the differences seen between alcohol-dependent subjects and healthy subjects can be attributed to alcohol effects per se. Focusing on individual differences in drug exposure within a population of alcohol-dependent subjects predominantly characterized by comorbid depression and anxiety (our inpatient population) avoids many such confounds.
Brain atrophy has been inferred from either sulcal volume
(21) or more specifically from the ratio of cerebral brain volume to total intracranial volume in investigations of healthy comparison subjects
(20), subjects with varying degrees of AIDS dementia
(22,
23), and subjects with Alzheimer’s disease
(24). Because cortical gray matter volume is tightly correlated with absolute numbers of neurons
(25), total and proportional gray matter calculated from segmented MRI images may reflect neuron loss.
In this report, we relate brain morphometric variables to drinking history as well as presence or absence of comorbid cocaine abuse or dependence diagnoses among male subjects with alcohol dependence. We hypothesized that drinking chronicity (lifetime exposure) would negatively correlate with gray matter volume and whole brain volume after age was controlled. We also hypothesized that alcohol-dependent subjects with comorbid cocaine use disorder would show a steeper age-mediated decline in white matter. As a positive control for nonspecific substance abuse-related effects, we also identified a group of alcohol-dependent subjects whose comorbid substance use disorder was limited to marijuana abuse or dependence. Because of scant neuroimaging data demonstrating any gross morphometric effects of delta-9-tetrahydrocannabinol (THC), the active ingredient of marijuana, together with in vitro data indicating a potential neuroprotective effect of THC
(26), we hypothesized that alcohol-dependent subjects with only a comorbid marijuana use disorder would not differ from subjects with alcohol dependence alone.
Method
All recruitment and testing procedures were reviewed and approved by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Institutional Review Board. After complete detoxification and withdrawal, experimental procedures (psychometric interviews and magnetic resonance imaging) were explained, and all patients provided written informed consent to participate.
Subjects
Male subjects with alcohol dependence (N=134; Caucasian: N=113, African American: N=17, Hispanic: N=4) were admitted at the National Institutes of Health Clinical Center in Bethesda, Md., for an inpatient alcoholism treatment program. Community-recruited male subjects (N=32; Caucasian: N=22, African American: N=5, Hispanic: N=2, other: N=3) with no history of significant medical illness or psychiatric disorders were also included for comparison. To help minimize cohort effects in a cross-sectional analysis, the age range of both alcoholic and healthy comparison subjects was restricted to 30–50 years. Alcohol-dependent patients whose IQ was below 80, who had gross neurological abnormalities, who had a history of psychotic symptoms, or who were not eligible for an MRI scan (e.g., metal implants) were excluded. All subjects were assessed with the Structured Clinical Interview for DSM-IV
(27), which confirmed that each patient met criteria for alcohol dependence and that no comparison subject met criteria for a psychiatric disorder.
For assessment of comorbid drug effects, alcohol-dependent subjects were assigned into one of three groups on the basis of substance use disorder comorbidity. Among alcohol-dependent subjects treated at NIAAA, the vast majority of patients with collateral substance use disorders are diagnosed with either cocaine or marijuana use disorders. Those with abuse or dependence of other substances (singly) were too rare for meaningful analysis and were excluded. The subjects with alcohol dependence alone (N=51) were those whose use of other psychoactive drugs (if any) did not meet substance use disorder criteria. The alcohol and marijuana use disorders group (N=33) comprised alcohol-dependent subjects who met DSM-IV criteria for marijuana abuse or dependence at some point in their life but never met criteria for substance use disorder for any other substance. Finally, the alcohol and cocaine use disorders group (N=50) comprised alcohol-dependent subjects who at some time in their life met criteria for either cocaine abuse or dependence. Only seven of the 50 alcohol-dependent subjects with a comorbid cocaine use disorder met criteria for only cocaine abuse or dependence and alcohol dependence. The majority (N=39) also met lifetime criteria for marijuana abuse or dependence, with 23 meeting lifetime criteria for two additional substance use disorders (besides alcohol and cocaine) such as sedatives or hallucinogens. The large majority of patients were also diagnosed with a comorbid mood disorder (41 of 51 subjects with alcohol dependence alone [80.4%], 25 of 33 alcohol-dependent subjects with comorbid marijuana use disorder [75.8%], and 41 of 50 alcohol-dependent subjects with comorbid cocaine use disorder [82.0%]). Patients had typically been drinking heavily for many years before seeking treatment, and it could not be confidently established whether the mood symptoms anteceded or were a consequence of the drinking. Therefore, differential diagnoses of primary or secondary mood disorder are not delineated.
A social worker administered a semistructured lifetime drinking history interview to each subject. Alcohol use history was divided into epochs of various use patterns according to each respondent’s history, and from these epochs we calculated three drinking history parameters: 1) age at onset of heavy drinking, defined a priori as the age at which the subject reported first consuming the equivalent of 90 drinks in a 1-month period; 2) years of heavy drinking, defined as the cumulative total contiguous or noncontiguous months during which the subject drank 90 drinks per month (note: since subjects often maintain this high a level of alcohol use for at least 12 consecutive months, months are summed into years); and 3) estimated lifetime alcohol consumption (in kg), which is a summation of all alcohol ingestion, including during periods where ingestion did not reach 90 drinks per month. Data were not normally distributed, so data were analyzed by rank. Subject age correlated positively with age at onset of heavy driinking (rs=0.35, p<0.0001) and mildly with years of heavy drinking (rs=0.17, p<0.05) but not with estimated lifetime alcohol consumption (rs=0.09, p=0.31).
MRI and Segmentation Analysis
Subjects underwent imaging with a 1.5-T MRI scanner (GE Medical Systems, Milwaukee) using a fast spoiled gradient/recall acquisition in the steady-state sequence. The scans were performed no sooner than 3 weeks after the subject’s last alcohol use. We used a gapless series of high-contrast, 2-mm thick T
1-weighted coronal images (repetition time=25 msec; inversion time=5 msec; echo time=16 msec). The images were acquired by using a 256×256 matrix with a 240×240-mm field of view. Each volumetric brain consisted of 124 coronal slices with voxel size of 0.9375×0.9375×2.0 mm. We manually separated intracranial tissue from the skull on coronal sections with a hand-driven cursor. The intracranial volume included the cerebrum and CSF spaces covering the cortex but excluded the cerebellum and CSF of the posterior fossa. Interrater reliability for manual identification of the intracranial volume of 10 randomly selected MRI volumes was high (intraclass correlation=0.97). Next, intracranial volume was automatically segmented into gray matter, white matter, sulcal CSF, and ventricular CSF according to a previously described and validated computerized method that used voxel intensity information to perform a nonsupervised K-means clustering procedure. (For further details, see reference
28.) Cerebral brain volume was calculated as the sum of gray matter and white matter.
We chose to include both intracranial volume and cerebral brain volume in our analyses because they are conceptually distinct. The skull (and thus intracranial volume) grows in tandem with the brain during childhood until about age 15, at which point the volume of the brain (but not the skull) begins to shrink in late adolescence and throughout adulthood
(29). Therefore, intracranial volume in an adult is a trait characteristic that reflects maximal brain growth during development, but brain volume in adults represents the outcome of both growth and atrophy processes. We also calculated intracranial-volume corrected volumes for gray matter and white matter (matter type divided by the difference of intracranial volume minus matter type) as an indirect measure of specific age-related atrophy of gray matter and white matter
(30). The additional terms in the denominator were included to control for compositional data (interrelated correlates), since intracranial volume is a function of the
sum of maximum CSF, white matter, and gray matter during development. Finally, we also calculated the gray matter/white matter ratio.
Results
Drinking History and Morphometric Measures
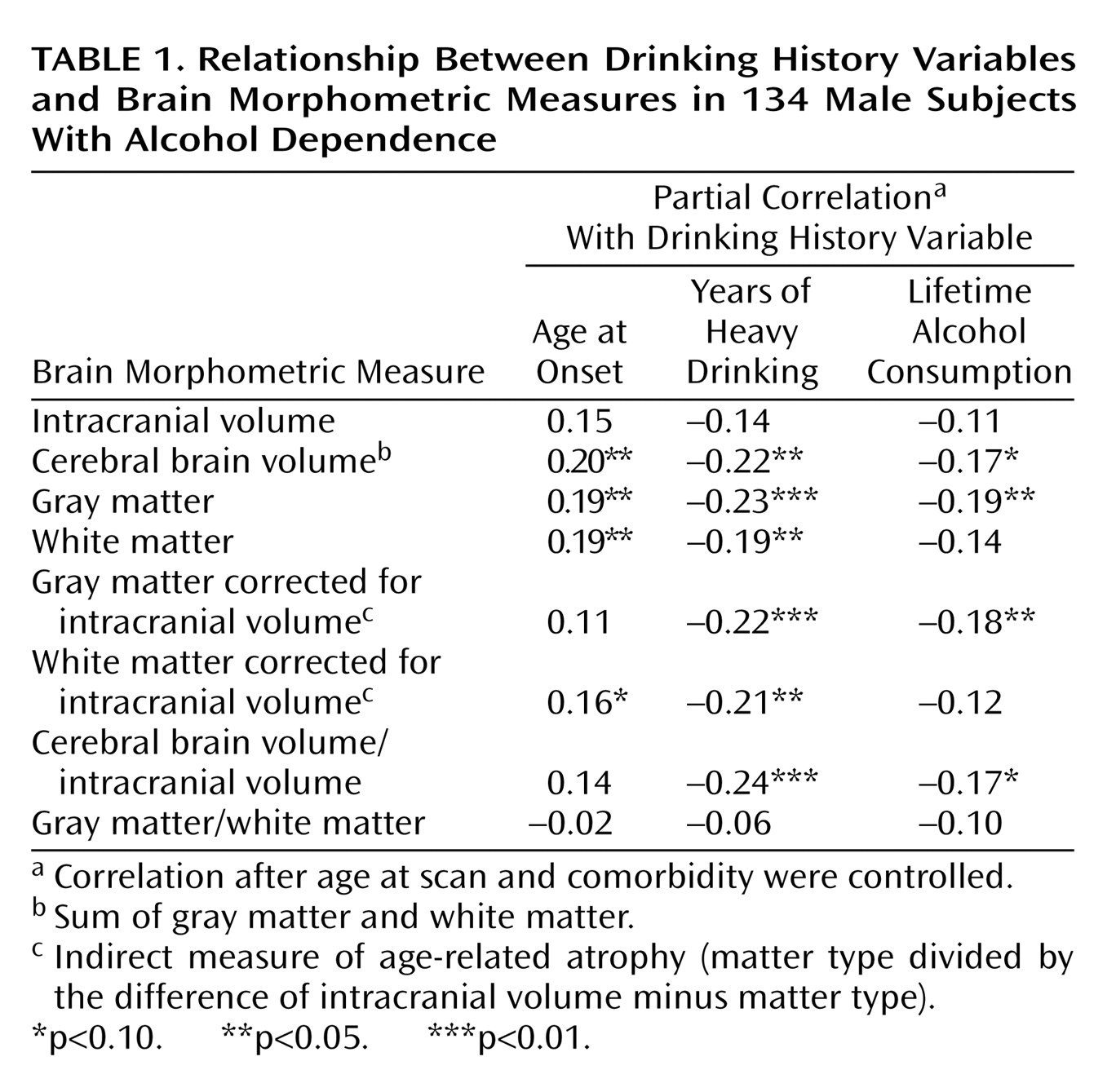
To disentangle independent effects (partial correlations) of age, drinking history, and comorbid marijuana or cocaine use disorder on volumetric measures, a series of multiple regression analyses were performed with age, comorbidity group, and each of the drinking history variables entered as independent variables, and each of the brain morphometric measures as the dependent variable. These analyses indicated that neither age, comorbidity status, nor any drinking history variable independently accounted for significant variance in intracranial volume.
With regard to total and proportional brain tissue volumes, age showed a negative partial correlation with brain volumetric measures in all analyses. Comorbid lifetime cocaine or marijuana use disorder, however, did not account for a significant portion of variance in any regression. In contrast, the number of years of heavy drinking showed a significant negative partial correlation with several brain volumetric measures after age and comorbidity were controlled (
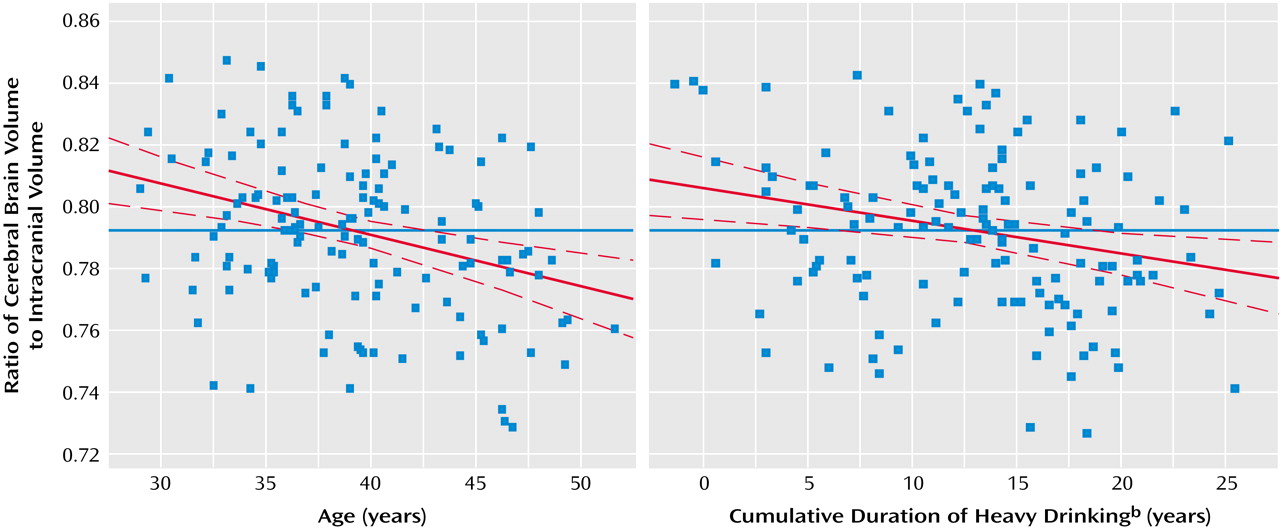
Table 1). To demonstrate minimal collinearity between age and years of heavy drinking as independent variables in model fitting, and to illustrate the independent relationships between both age and years of heavy drinking with brain atrophy, we used the model-fitting module of the statistical package JMP-SAS (SAS Institute; Cary, N.C.) to create leverage plots whose slopes represent the partial correlations between the cerebral brain volume/intracranial volume ratio and both age and years of heavy drinking (
Figure 1).
Demographic, Drinking Measure, and Morphometric Differences by Comorbidity Group
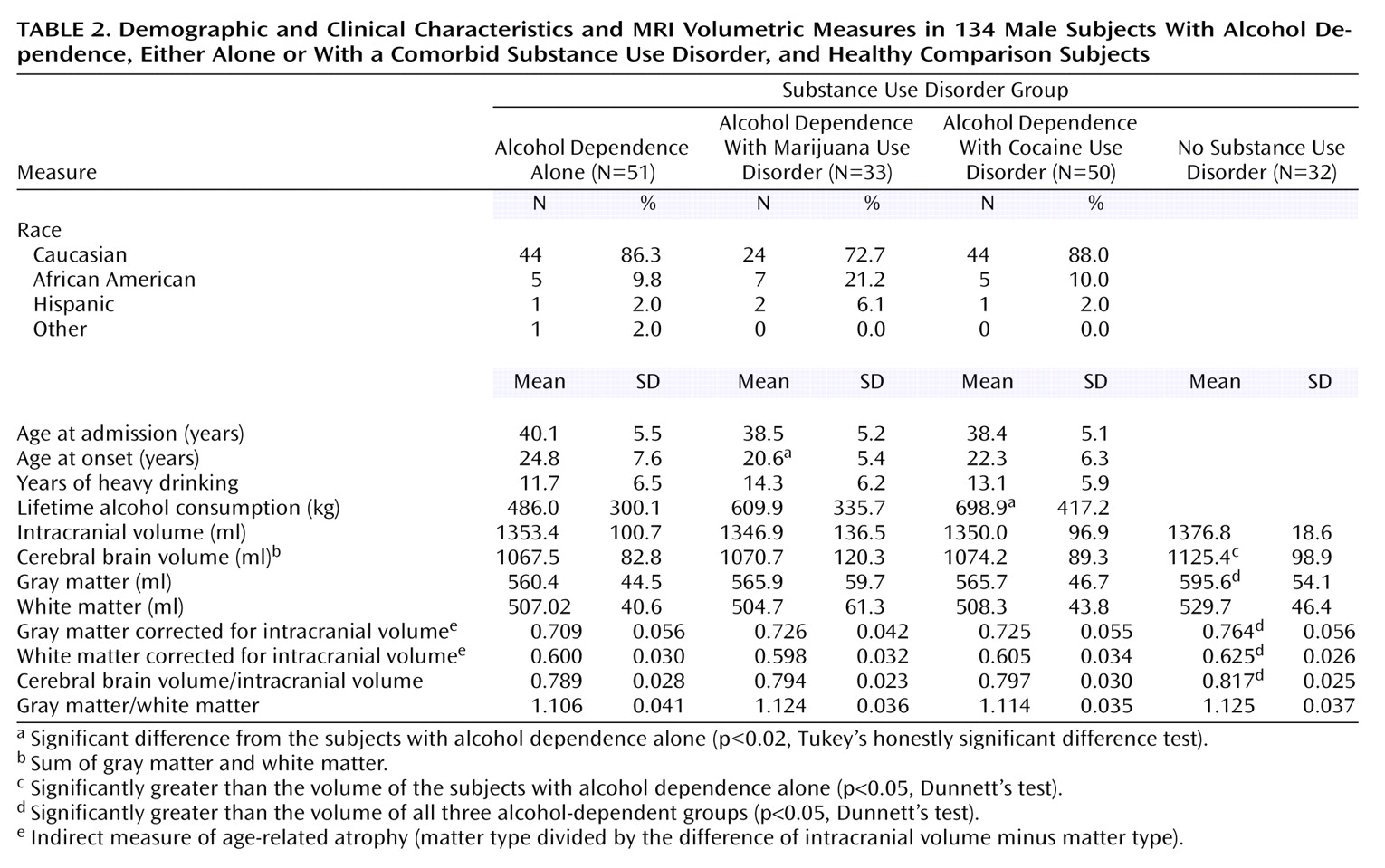
The three alcohol-dependent groups did not significantly differ in race or age (
Table 2). Tukey’s honestly significant difference tests indicated that in relation to those subjects with alcohol dependence alone, the alcohol-dependent subjects with a comorbid marijuana use disorder had a significantly earlier age at onset of heavy drinking (>90/month), and the alcohol-dependent subjects with a comorbid cocaine use disorder consumed significantly more alcohol. There were no significant group differences, however, in total and proportional brain volumes between the three groups, and mean intracranial volume was nearly identical across groups. While this report focuses on sources of volumetric differences among alcohol-dependent subjects, and comparisons between alcohol-dependent subjects and healthy subjects are presented in detail elsewhere, to put the brain volume measures of the alcohol-dependent subjects into broader perspective, we also present data from the 30–50-year-old male comparison subjects (
Table 2). Parenthetically, analyses of variance followed by Dunnett’s post hoc comparisons with a healthy group indicated that all three alcoholic groups had significantly lower raw and intracranial volume-corrected brain volume, gray matter volume, and white matter volume than did the comparison subjects.
Bivariate Correlations Between Age and Brain Measures Across Comorbidity Groups
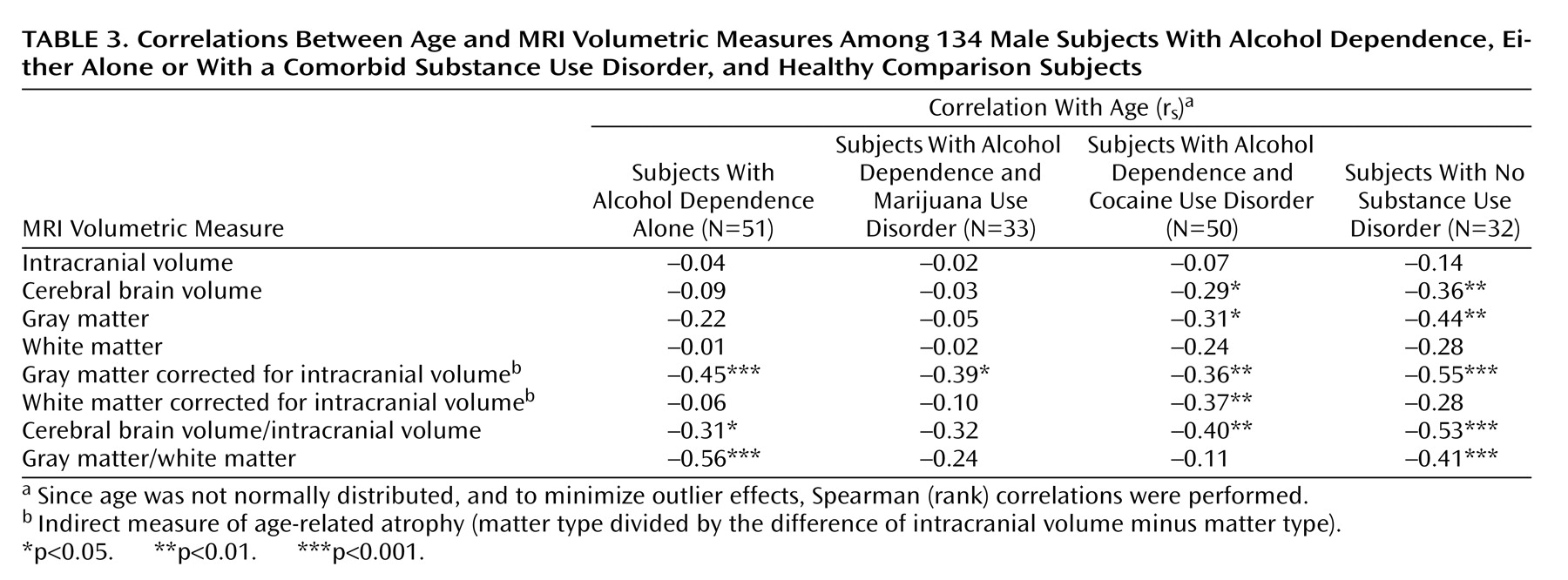
To assess whether alcohol-dependent subjects with and without comorbid marijuana or cocaine use disorders differed in how age correlated with brain volumes, we correlated age-by-brain volumes in each comorbidity group separately. Since age was not normally distributed, and to minimize outlier effects, Spearman (rank) correlations were performed (
Table 3). We note that alcohol-dependent subjects treated at NIAAA often began heavy alcohol use in adolescence and were already showing significantly altered brain morphology by age 30 relative to healthy comparison subjects (data not presented), resulting in generally blunted age correlations with brain volumes later in adulthood compared with similar correlations in healthy subjects. The relatively steeper age correlations with volumetric measures in comparison subjects are presented for reference (
Table 3). Age correlated negatively with intracranial volume-corrected gray matter in all three alcohol-dependent groups, and age also correlated negatively with intracranial volume-corrected cerebral brain volume in subjects with alcohol dependence either alone or with a comorbid cocaine use disorder (with a nonsignificant negative correlation seen for alcohol-dependent subjects with comorbid marijuana use disorder). The correlation between age and intracranial volume-corrected white matter was significant only in alcohol-dependent subjects with a comorbid cocaine use disorder, and the gray matter/white matter ratio decreased with age only in the subjects with alcohol dependence alone. There was no correlation between age and intracranial volume in any group.
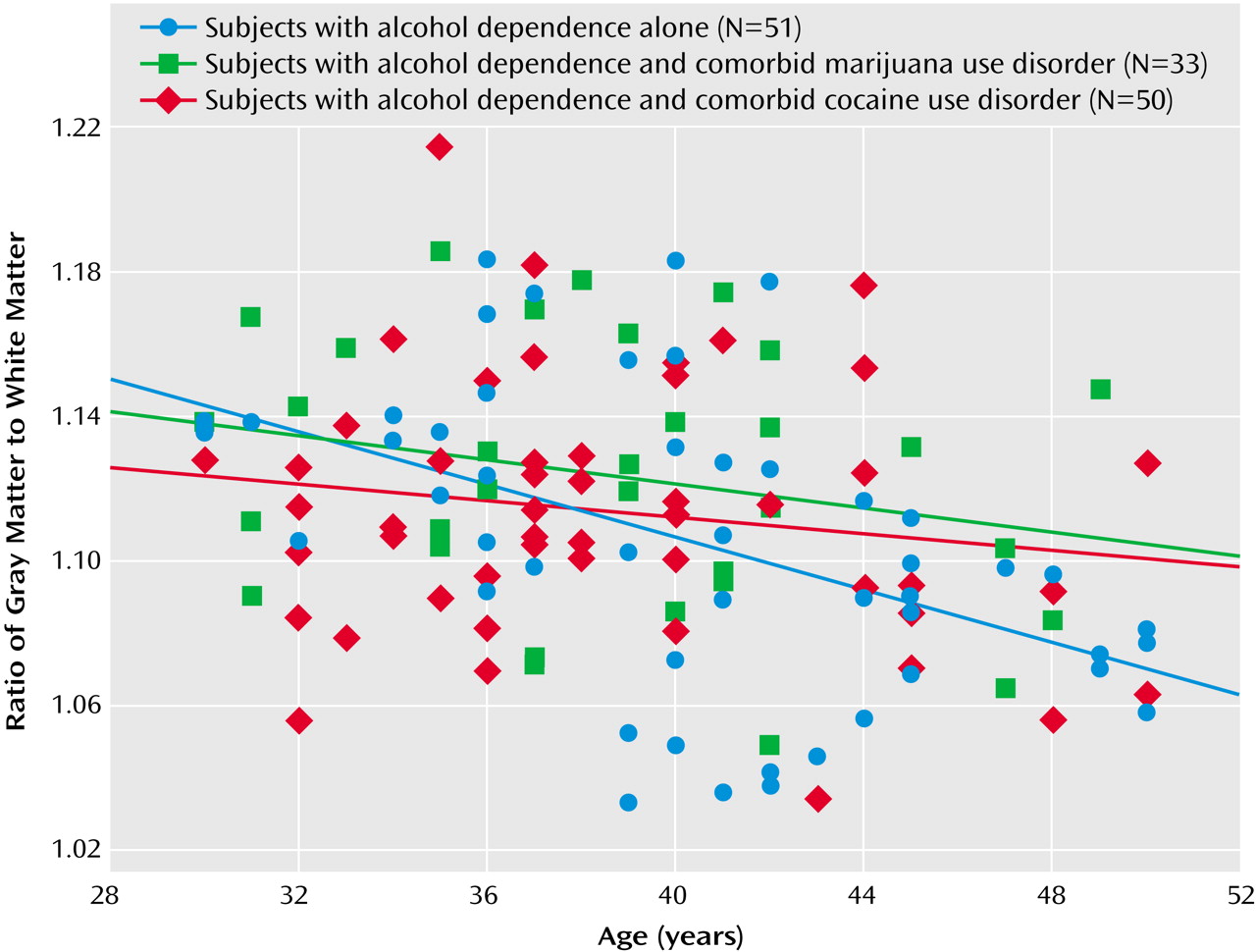
We also compared correlations between proportional brain volumes with age by using one-tailed Fisher z tests with an a priori expectation of more severe white matter atrophy in alcohol-dependent subjects with comorbid cocaine use disorder. The negative correlation between age and the intracranial volume-corrected white matter volume tended to be steeper in the alcohol-dependent subjects with comorbid cocaine use disorder than in the subjects with alcohol dependence alone (p=0.061). The subjects with alcohol dependence alone demonstrated a steeper negative correlation between age and the gray matter/white matter ratio than did the alcohol-dependent subjects with comorbid cocaine use disorder (p=0.006) and, to a lesser extent, the alcohol-dependent subjects with comorbid marijuana use disorder (p=0.05). For illustrative purposes, linear fits of age-by-gray matter/white matter ratio for each group are overlaid in
Figure 2.
Discussion
Previous cross-sectional
(2,
30) and longitudinal
(3,
31) volumetric MRI comparisons between alcohol-dependent subjects and healthy subjects have indicated that individuals diagnosed with alcohol dependence show greater age-related brain atrophy. Here we extend these findings by characterizing individual differences within a population of alcohol-dependent subjects. Specifically, the total cumulative year equivalents of consumption of 90 or more drinks in a month was negatively correlated with total and intracranial volume-corrected brain volumes after the effects of age were controlled. This relationship was not as consistent with regard to total lifetime drinks (which includes consumption both inside and outside the context of clinically significant heavy drinking). It may be that brain volume is particularly affected by periods of intense alcohol exposure. Alternatively, subject report of the precise quantity of drinking may have been more prone to inaccurate recollection compared with the recollection of the duration of extended periods of heavy alcohol use, rendering total lifetime drinks a less valid measure.
Group comparisons indicated that alcohol-dependent subjects with a lifetime cocaine use disorder diagnosis drank more than subjects with alcohol dependence alone but did not show smaller whole or proportional brain volumes in bivariate analysis (
Table 2). In particular, alcohol-dependent subjects with comorbid cocaine use disorder did not have smaller age- and drinking history-adjusted cerebral brain volume/intracranial volume ratios relative to subjects with alcohol dependence alone, and a comorbid substance use disorder diagnosis itself did not account for a significant portion of variance in any regression when age and alcohol exposure variables were included as covariates. This indicates that a comorbid cocaine use disorder (as with comorbid marijuana use disorder) does not further affect brain MRI morphometry measures above and beyond age and alcohol consumption.
A handful of imaging reports have indicated that cocaine use is neurotoxic to the adult brain. We provide here limited evidence that comorbid cocaine and alcohol use disorders (but not marijuana use disorder alone in conjunction with alcoholism) may accelerate white matter shrinkage with age relative to alcohol dependence alone. The alcohol-dependent subjects with a lifetime history of cocaine use disorder tended to have the highest (negative) correlation between age and white matter as a proportion of intracranial volume. This trend toward increased white matter degeneration in the whole brain is in concordance with the analyses of Bartzokis et al.
(15), who reported that healthy subjects showed an increase in frontal and temporal lobe white matter volume from age 19 to 47, but cocaine-dependent subjects (about 40% of whom had a lifetime diagnosis of alcohol dependence) did not. It is possible the increased white matter reduction with age in cocaine-abusing alcohol-dependent subjects may have resulted from neurotoxic effects of cocaethylene
(9).
Mean intracranial volume was nearly identical among the groups of alcohol-dependent subjects. This suggests that total and proportional brain volumes, as well as differential patterns of age-related atrophy, were a consequence of chronic drug use effects occurring after maximal brain growth. While a portion of adolescents binge drink (12%
[32]) or use cocaine (less than 2%
[33]) before age 15, to potentially stunt maximal brain and skull growth, we note that the age of first consumption of 90 or more drinks per month was below 15 in only seven of 134 subjects.
Some features of this data set complicate drawing conclusions about collateral drug effects on brain volumes in subjects with alcohol dependence. First, the data were highly variable, with alcohol use history parameters and even age counting for modest amounts of variance in the volumetric measures. Second, this was a cross-sectional analysis that may have been confounded by cohort effects, such as changes in drug use patterns over the past 30 years. Third, the alcohol-dependent subjects with comorbid cocaine use disorder may have been more antisocial and may have underreported the severity and quantity of their alcohol consumption, such that “comorbid cocaine” effects were simply a more severe manifestation of chronic high-dose alcohol damage. However, one recent methodological study indicated no discrepancy between alcohol-dependent subjects with and without comorbid cocaine dependence in the concurrence between self-report and collateral report of their alcohol use
(34). Nevertheless, the volumetric characteristics of the alcohol-dependent subjects with comorbid cocaine use disorder may have been a consequence of greater alcohol neurotoxicity. Most important, the alcohol-dependent subjects with comorbid cocaine use disorder were admitted for treatment having declared problems with alcohol, not cocaine, as their primary motivation for seeking therapy. Consequently, the total amount of cocaine used by these subjects (data not collected) may have been less, on average, than that seen in a population of patients referred specifically for cocaine problems. Finally, we note that while substance use disorder comorbidity seemed to have no additive effect on brain volumes calculated from segmentation of MRI slices, cocaine or marijuana may have affected brain morphology in a fashion not detectable with the morphometric methods we employed. For example, we would not detect increases in the volume of white matter hyperintensities visible on T
2-weighted images.
In conclusion, this study 1) used a sample considerably larger than previously published work examining brain volume and cocaine use disorder, 2) measured the entire brain instead of a portion, and 3) explored finer-grained differences among alcohol-dependent subjects in terms of alcohol and other drug exposure. Our data provide some support of our hypothesis of more severe age-related white matter atrophy in alcohol-dependent male subjects with comorbid lifetime diagnoses of cocaine abuse or dependence compared with male subjects with alcohol dependence alone. However, alcohol-dependent subjects with cocaine use disorders on the whole did not have significantly less white matter or gray matter than alcohol-dependent subjects without cocaine use disorders. It appears that the predominant correlate of brain atrophy in these alcoholic patients was chronicity of heavy alcohol use and not presence or absence of a collateral substance use disorder. Future cross-sectional analyses should include older patients with comorbid cocaine or marijuana use disorder as well as alcohol-dependent women. Of additional interest would be longitudinal experimental designs, with repeated MRI scans and prospective tracking of alcohol, cocaine, and marijuana use to better disentangle drug effects on volumetric brain measures over time.