Postmortem studies have indicated that morphological brain changes during the early, preclinical stages of Alzheimer’s disease almost selectively involve the parahippocampal gyrus
(1,
2). However, previous studies using magnetic resonance imaging (MRI) for volumetric investigations of the medial temporal lobe in elderly subjects with mild cognitive deficits, who are generally considered to have an increased risk for developing Alzheimer’s disease, have yielded inconclusive results
(3–
12). The diversity of findings may be related partly to the criteria used to define mild cognitive deficits, insofar as most previous MRI studies refer to amnestic forms, such as age-associated memory impairment
(13) and mild cognitive impairment
(14). An alternative approach for defining mild cognitive deficits makes use of the concept of aging-associated cognitive decline
(15). In contrast to age-associated memory impairment, aging-associated cognitive decline is defined by age-adjusted norms and considers deficits in a broad range of cognitive domains.
The present study examined regional brain volumes in a group of otherwise healthy elderly subjects with mild cognitive deficits defined according to the criteria for aging-associated cognitive decline. On the basis of previous neuropathological findings, we expected these subjects to have structural volume deficits in the medial temporal lobe regions, particularly the parahippocampal gyrus.
Method
Subjects with mild cognitive deficits and cognitively intact comparison subjects were recruited among a subset of subjects from the Heidelberg area who participated in a population-based, interdisciplinary, longitudinal study of adult development (N=252 subjects born from 1930 to 1932). The health of all subjects was screened by means of medical history, physical examination, ECG, and laboratory testing. Cognitive performance was assessed with an extensive neuropsychological test battery. A detailed description of the study protocol for the longitudinal study and a description of the neuropsychological test battery have been published previously
(16,
17).
Subjects with aging-associated cognitive decline were identified according to the following criteria, developed by a work group of the International Psychogeriatric Association
(15):
1. Performance on a standardized test of cognition at least one standard deviation below the age-adjusted norm in at least one of the following domains: learning and memory, language, attention and cognitive speed, and visuoconstructional abilities.
2. Exclusion of any medical, neurological, or psychiatric disorder that could produce cognitive deterioration, as determined by the medical history and/or clinical examination.
3. Normal activities of daily living and exclusion of dementia identified according to the DSM-IV criteria.
Thirty-five of the 47 subjects who fulfilled the criteria for aging-associated cognitive decline at baseline as well as a corresponding number of cognitively unimpaired comparison subjects (matched for age, gender, and educational level) consented to participate in an MRI investigation that took place 30–50 months after the first visit. Thus, 71 subjects in the original interdisciplinary longitudinal study of adult development were reexamined clinically, neuropsychologically, and by MRI at follow-up.
Subjects with significant indications of cerebrovascular disease revealed by MRI, emergence of a neurological/medical condition sufficient to cause cognitive decline, or incomplete/inappropriate MRI data sets (e.g., withdrawal due to claustrophobia, motion artifacts) were excluded. There were no significant differences in neuropsychological and sociodemographic characteristics between those who were excluded and those who remained in the analysis. Of the remaining 43 subjects, 21 fulfilled the criteria for aging-associated cognitive decline at follow-up, and 22 constituted the cognitively unimpaired comparison group.
As an additional reference group, 12 patients with mild Alzheimer’s disease (diagnosed according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
[18]) were recruited from the section on geriatric psychiatry at the University of Heidelberg. The 12 patients were matched for age and gender with the other study groups. The Global Deterioration Scale
(19) was used to compare the degree of cognitive impairment among the three study groups.
The MRI data were acquired with a 1.5-T Siemens scanner (Erlangen, Germany) by using a three-dimensional magnetized prepared rapid gradient echo sequence for the T
1-weighted slices (TR=10 msec, TE=4 msec) and a three-dimensional reverse fast imaging with steady precession sequence (TR=17 msec, TE=7 msec) for the T
2-weighted slices, generating 128 coronal slices for each sequence (slice thickness=1.8 mm). Volumetric measurements included the total intracranial volume, the whole brain volume, and the CSF volume, as well as volumes of the frontal and temporal lobes and the hippocampal formation bilaterally, as described previously
(20,
21). In addition, volume measures of the parahippocampal gyrus were sampled on the same slices as the hippocampal measures. Although total intracranial volume and whole brain volume were determined by using semiautomatic segmentation algorithms (r=0.98 [intercorrelation coefficient], p<0.0005, N=20), volumes of the other structures were evaluated by manual tracing (0.96<r<0.99 [intercorrelation coefficient], p<0.0005, N=16). (A detailed protocol of measurements will be sent on request.) All volumetric data were corrected by dividing each raw value by the subject’s total intracranial volume. Analyses of variance with Duncan’s tests were calculated to compare the data between the diagnostic groups. Stepwise linear regression was used to assess the relationship between the extent of global cognitive impairment (Global Deterioration Scale score), level of education, and corrected morphometric values within the aging-associated cognitive decline group.
Results
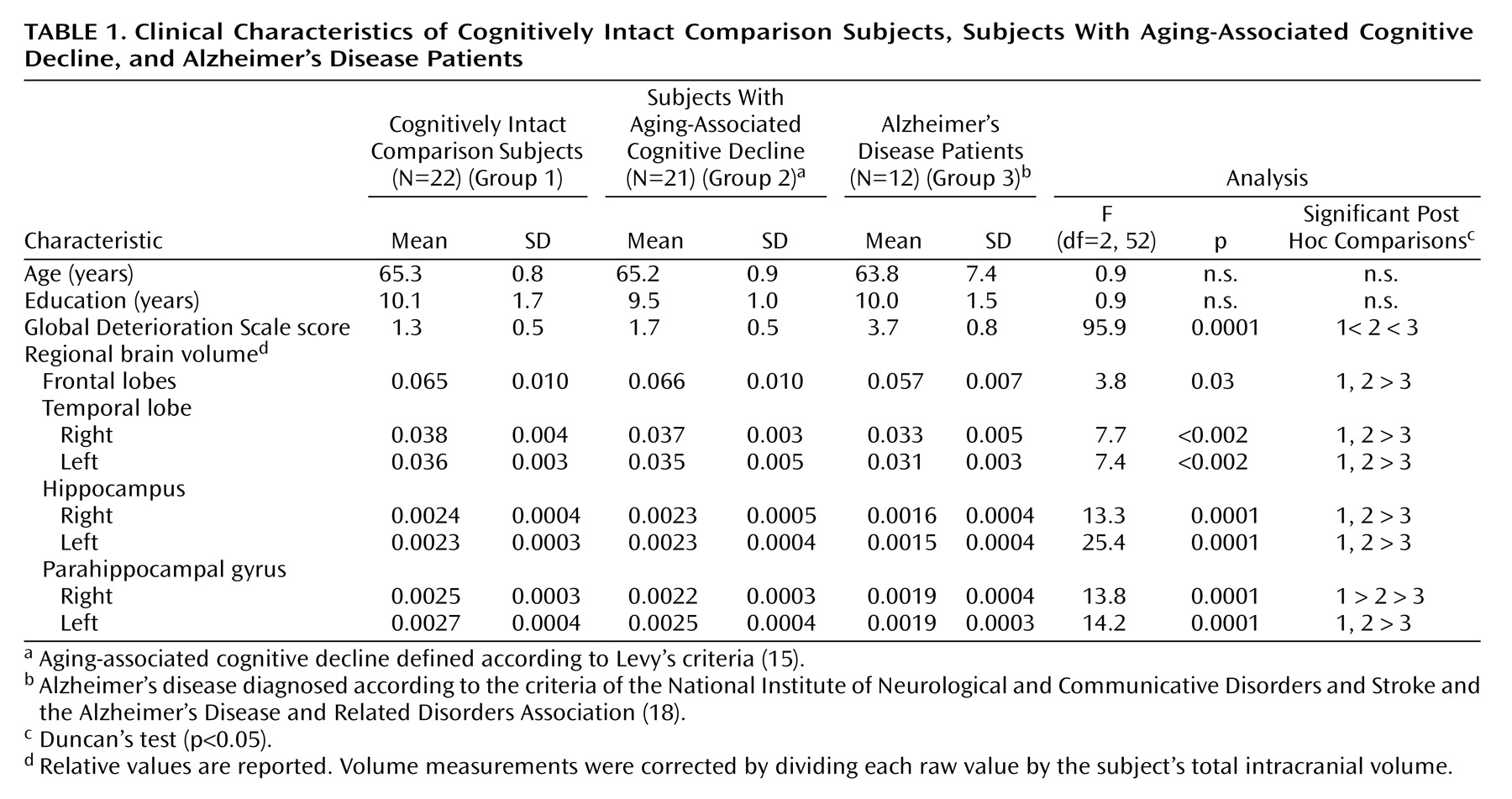
Age, gender distribution, level of education, and total intracranial volume did not differ significantly between the groups. Subjects with aging-associated cognitive decline had a significantly lower mean volume of the right parahippocampal gyrus (–12%) (F=13.8, df=2, 52, p=0.0001) than the comparison group (
Table 1). The mean volume of the left parahippocampal gyrus was 7% smaller in the aging-associated cognitive decline group than in the comparison group, although this difference did not reach statistical significance. By contrast, Alzheimer’s disease patients had a significantly lower volume of the bilateral parahippocampal gyrus (right: F=13.8, df=2, 52, p=0.0001; left: F=14.2, df=2, 52, p=0.0001), relative to both the aging-associated cognitive decline group (right: 13.6% lower in the Alzheimer’s disease patients; left: 24.0% lower) and the comparison group (right: 24.0% lower in the Alzheimer’s disease patients; left: 29.6% lower).
The whole brain volume, the CSF volumes, and the frontal, temporal, and hippocampal volumes of the aging-associated cognitive decline subjects were not significantly different from those of the comparison subjects. In contrast, the Alzheimer’s disease patients were characterized by significantly smaller frontal as well as hippocampal and temporal lobe volumes bilaterally, relative to both the group with aging-associated cognitive decline and the comparison group (3.8≤F≤25.4, df=2, 52, 0.0001≤p≤0.03).
In a stepwise linear regression analysis, only the right parahippocampal gyrus volume remained in the regression model for the extent of global cognitive impairment of the aging-associated cognitive decline subjects as indicated by the Global Deterioration Scale (R=0.53, F=7.4, df=1, 20, p<0.05).
Discussion
To our knowledge, this is the first MRI study demonstrating a significant deficit in the volume of the parahippocampal gyrus in subjects with aging-associated cognitive decline. Consistent, although much more pronounced, changes were found in the Alzheimer’s disease patients, indicating that, with respect to parahippocampal gyrus volume, the subjects with aging-associated cognitive decline took an intermediate position between the Alzheimer’s disease patients and the cognitively intact comparison subjects. Our results suggest that cerebral volume deficits in subjects with aging-associated cognitive decline preferably, and possibly selectively, involve the parahippocampal gyrus, leaving other regions of the brain relatively unaffected.
In view of the neuropathological evidence, it is conceivable that the observed parahippocampal gyrus volume deficit reflects degenerative atrophic changes, as described for the early preclinical (transentorhinal) stages of Alzheimer’s disease
(1,
2). This assumption is supported by the significant association between parahippocampal gyrus volumes and severity of cognitive deficits in the aging-associated cognitive decline group, a finding in line with the view that pathological alterations in the parahippocampal gyrus leading to a disconnection of the hippocampus from neocortical association areas represent the neuroanatomical correlate of cognitive dysfunction in early dementia
(2,
22).
Hitherto, structural MRI studies have revealed only inconclusive evidence linking medial temporal lobe atrophy with mild cognitive deficits. The divergent results may be related to aspects of study subject selection. In the majority of previous studies—in particular those yielding negative results—subjects were selected on the basis of the concept of age-associated memory impairment
(4–
6,
9), which does not consider age-adjusted norm values when defining cognitive deficits. In addition, age-associated memory impairment refers solely to mnestic functions and might exclude at-risk subjects for whom the development of dementia is preceded by a disturbance of other cognitive domains. In contrast to age-associated memory impairment, aging-associated cognitive decline includes not only memory problems but also performance deficits in any other principal domain of cognition. Accordingly, the concept of aging-associated cognitive decline takes into account the heterogeneity of neuropsychological deficits potentially preceding the development of Alzheimer’s disease. Indeed, impairment defined according to the criteria for aging-associated cognitive decline could be associated with a higher risk for subsequent dementia than impairment defined according to previously used criteria
(23).
In summary, we conclude that quantitative MRI has the potential to detect the earliest morphological alterations of the medial temporal lobe in individuals who are at risk for developing the full clinical picture of Alzheimer’s disease subsequently. These alterations appear to affect selectively the parahippocampal gyrus. Such findings could be used to facilitate the preclinical diagnosis of Alzheimer’s disease.