The neuroanatomical pathology of autism is still poorly understood. Changes in the cerebellum, hippocampus, amygdala, basal ganglia, cerebral ventricles, and planum temporale have all been described
(1–
4), although some of the changes have not been replicated in follow-up studies. Medial temporal lobe structures such as the hippocampus and amygdala have been of particular interest because these limbic structures have been proposed to underlie key behavioral dysfunctions in autism
(5). Bauman and Kemper
(1) first reported evidence of higher cell packing density and smaller neuronal size in the hippocampus, amyg-dala, and entorhinal cortex and in several other structures in a single case autopsy study. These findings were subsequently replicated in a larger sample
(6).
Noninvasive magnetic resonance imaging (MRI) studies have also been performed on the hippocampus and amygdala. The results for both structures are equivocal. Several studies of hippocampal volume have failed to find differences in children and adults with autism
(7–
10). There have been reports of smaller hippocampal volume
(11,
12) as well as larger hippocampal volume
(13) in autism, although the latter finding was true only before statistical correction for total brain volume. As with the hippocampus, some studies of the amygdala have had negative findings
(10) and others have reported smaller amygdala volume
(11) and larger amygdala volume
(9,
13) in autism.
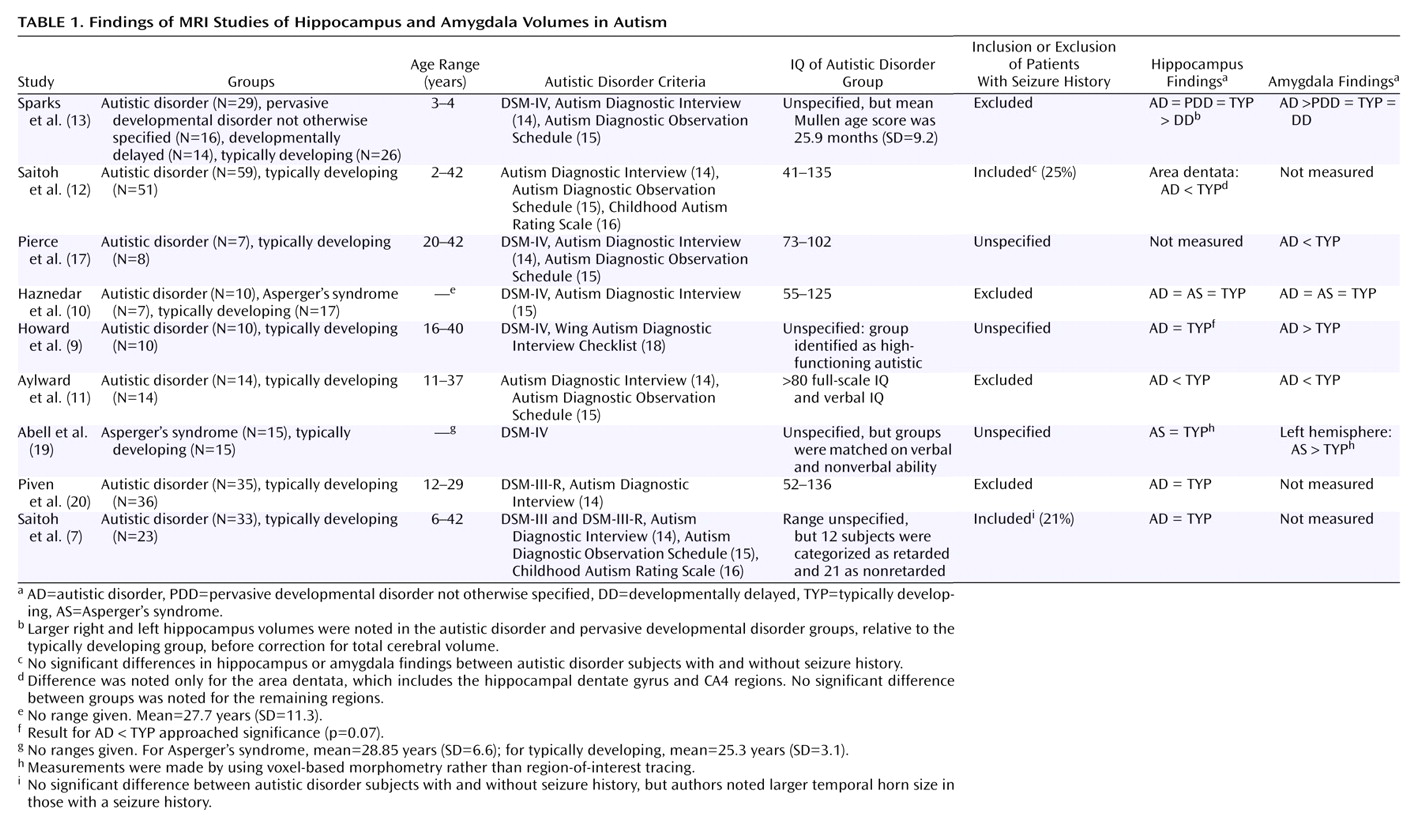
Table 1 summarizes the published findings on hippocampus and amygdala volumes in autism.
To our knowledge, no studies of the medial temporal lobe in relatives of individuals with autism have been reported. Studies of unaffected relatives can yield information on which anatomical components of the disorder may be heritable. This information in turn may help resolve some ambiguity surrounding the direction (smaller or larger) of the deficit, if any. Studies of unaffected relatives also largely avoid confounding issues such as intellectual dysfunction, disorder history, and, in the case of parents, child and adolescent development.
For this study, we examined hippocampus and amygdala volumes in clinically unaffected parents of children with autistic disorder, adults with autistic disorder, and adults with no personal or familial history of autism. The current study was an attempt to establish the familiality of differences in medial temporal lobe structures that have been previously implicated in autistic disorder. We reasoned that a volumetric difference in autism would be most pronounced in the affected adults and somewhat less so in the unaffected parents of children with autism, relative to the comparison subjects, given the high genetic contribution to the disorder suggested by twin studies
(21). This approach is similar to that of MRI studies of unaffected first-degree relatives of schizophrenia probands (e.g., references
22,
23), which have suggested that smaller hippocampal volume may be a genetically mediated neurobiological risk factor for schizophrenia
(24). Given the ambiguity of the prior work on medial temporal lobe structures in individuals with autism, we could not specify a priori in what direction (smaller or larger) the differences, if seen, would appear.
Results
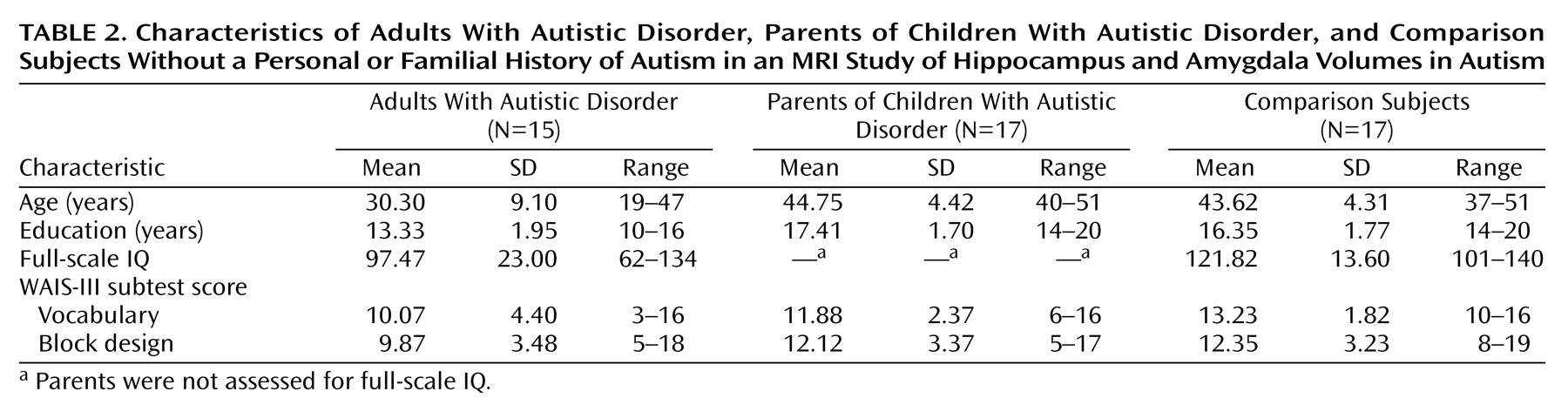
There was an overall age difference between the groups (F=26.25, df=2, 46, p<0.0001). This difference was entirely due to the inclusion of the adults with autistic disorder, who as a group were significantly younger than both the parents of children with autistic disorder (p<0.0001, Fisher’s least significant difference test) and the comparison group (p<0.0001, Fisher’s least significant difference test). The age-matched parents of children with autistic disorder and the comparison subjects did not differ in age (p=0.60, Fisher’s least significant difference test), as expected. The education level of the participants was also different between groups (F=21.66, df=2, 46, p<0.0001) and was significantly lower for the adults with autistic disorder than for the other two groups (p<0.0001 and p<0.0001, respectively, Fisher’s least significant difference test) but did not differ between the comparison subjects and the parents of children with autistic disorder (p=0.93, Fisher’s least significant difference test). The WAIS-III vocabulary scaled scores also differed between groups (F=4.46, df=2, 46, p<0.02). For the vocabulary subtest, the adults with autistic disorder and the comparison subjects differed (p<0.005, Fisher’s least significant difference test); the difference between the adults with autistic disorder and the parents of children with autistic disorder did not reach significance (p<0.10, Fisher’s least significant difference test). The parents of children with autistic disorder and the comparison subjects did not differ in vocabulary scores (p=0.19, Fisher’s least significant difference test). For the WAIS-III block design subtest, the groups did not differ (F=2.61, df=2, 46, p>0.05). For full-scale IQ, the adults with autistic disorder differed significantly from the comparison group (t=3.70, df=30, p<0.001). Group means and standard deviations for these measures are provided in
Table 2.
The correlation analyses of the relationships between the demographic variables (age, education level, WAIS-III full-scale IQ, performance on the WAIS-III vocabulary and WAIS-III block design subtests) and the morphometric variables used a Bonferroni corrected alpha of p=0.002. Given that correction, the only significant correlations were between age and left and right hippocampal volumes (N=49, r=–0.51 and r=–0.46, respectively). Total brain volume correlated only with the other four volumetric measures: left hippocampus volume (r=0.55), right hippocampus volume (r=0.51), left amygdala volume (r=0.54), and right amygdala volume (r=0.47). Therefore, age and total brain volume were both used as covariates in analyses involving the hippocampus, and total brain volume was used as a covariate in analyses involving the amygdala.
Gender was also examined in relation to MRI volume measures. Left hippocampal volume was significantly larger in men (mean=4.71 ml, SD=0.43) than in women (mean=4.07 ml, SD=0.33) (t=5.62, df=47, p<0.0001). Right hippocampal volume was also larger in men (mean=4.73 ml, SD=0.47) than in women (mean=4.10 ml, SD=0.33) (t=5.26, df=47, p<0.0001). Both the left amygdala (men: mean=3.71 ml, SD=0.34; women: mean=3.34 ml, SD=0.34) and the right amygdala (men: mean=3.75 ml, SD=0.35; women: mean=3.38 ml, SD=0.30) were significantly larger in the men than the women (t=3.76, df=47, p<0.001, and t=3.92, df=47, p<0.001, respectively). Finally, total brain volumes differed significantly between men (mean=1302.70 ml, SD=113.03) and women (mean=1164.21 ml, SD=113.51) (t=4.21, df=47, p<0.001). Gender was therefore used as a covariate in all group comparisons of brain volumes.
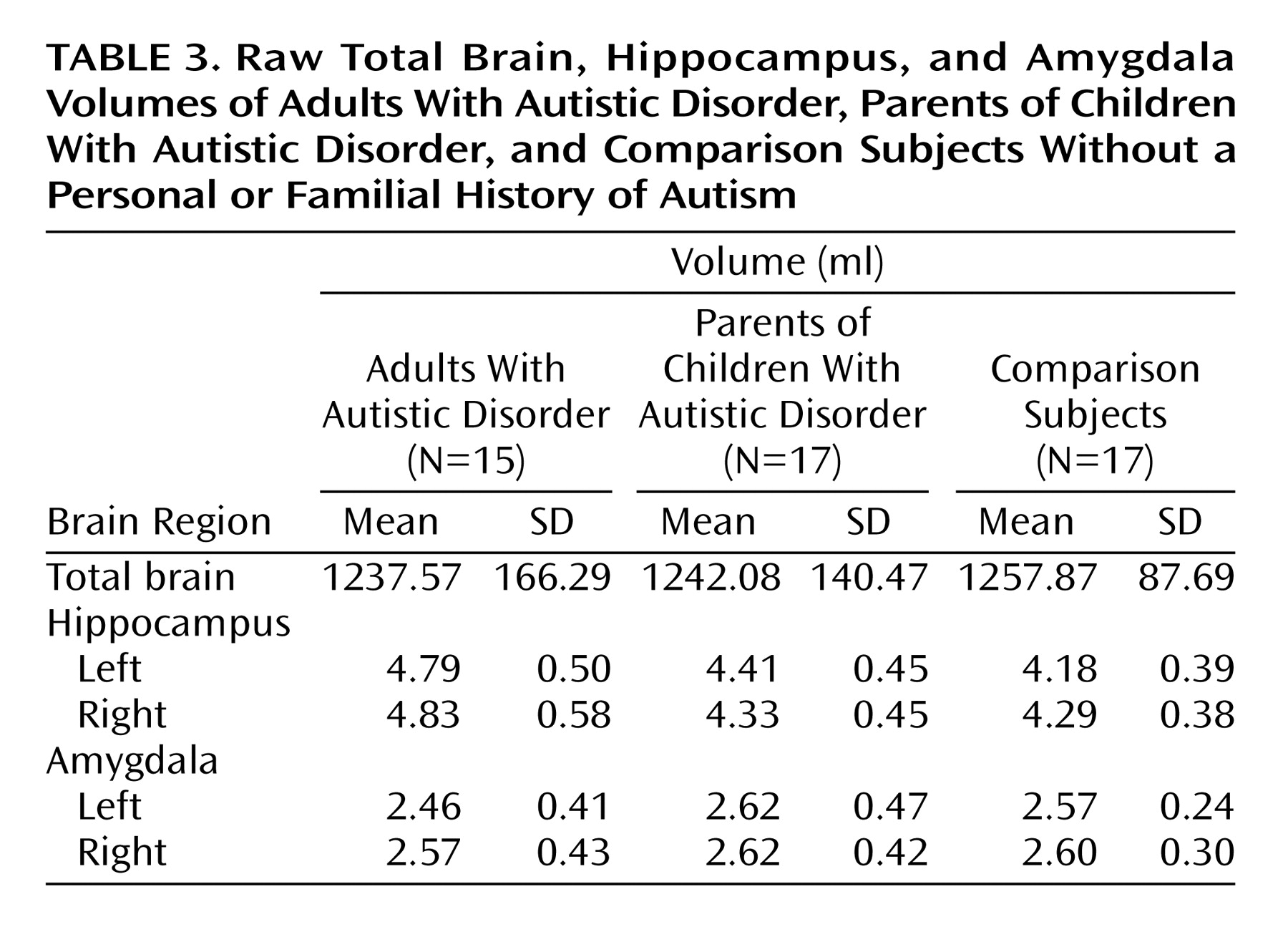
Although the mean total brain volumes (analyzed with a one-way ANCOVA with group as the single factor and gender as a covariate) for the adults with autistic disorder and the parents of children with autistic disorder (mean=1237.57 ml, SD=166.29, and mean=1242.08 ml, SD=140.46, respectively) were slightly smaller than the total mean brain volume for the comparison subjects (mean=1257.87 ml, SD=87.69), the difference did not reach statistical significance (F=2.34, df=2, 45, p=0.11).
In the separate analyses of left and right hippocampal volumes, the volume of the left hippocampus differed between groups (F=4.63, df=2, 43, p<0.02). Post hoc analyses revealed that the left hippocampus was significantly larger in the adults with autistic disorder than in both the parents of children with autistic disorder (p=0.002, Fisher’s least significant difference test) and the comparison group (p<0.0001, Fisher’s least significant difference test). The left hippocampus of the parents of children with autistic disorder was also significantly larger than that of the comparison subjects (p<0.05, Fisher’s least significant difference test). The volume of the right hippocampus did not differ significantly between groups (F=2.53, df=2, 43, p=0.09). The group means and standard deviations for the raw hippocampal volumes are provided in
Table 3.
In the separate analyses of the left and right amygdala volumes, the volume of the left amygdala differed between groups (F=3.42, df=2, 44, p<0.05). Post hoc analyses revealed no groupwise differences, which most likely indicated that adjustment for the covariates of total brain volume and gender was necessary to achieve significance in the overall analysis. Indeed, reanalysis of the results without gender as a covariate yielded no significant difference between groups (F=0.95, df=2, 45, p>0.05). No significant differences were observed between groups for the right amygdala (F=1.13, df=2, 44, p>0.05), with or without the covariates included in the model. The group means and standard deviations for the raw amygdala volumes are provided in
Table 3.
Discussion
We found larger left hippocampal volume in biological parents of children with autism, relative to age-matched healthy comparison subjects. Adults with autistic disorder were also included in the study to establish the directionality of differences in hippocampal volume that would suggest a genetic component if the difference also appeared in the parent group. The adults with autistic disorder showed larger left hippocampal volume, relative to both the comparison group and the parent group. Larger hippocampal volumes have been reported in other neurodevelopmental disorders such as fragile X syndrome
(32,
33). This finding is provocative, in that fragile X syndrome is one of the few known genetic etiologies for autism. As many as 15% of children with fragile X syndrome, caused by a CGG trinucleotide expansion of the
FMR1 gene on the X chromosome, also meet the diagnostic criteria for autistic disorder
(34). Reiss and colleagues
(32,
33) have also found age-dependent larger hippocampal volumes in subjects with fragile X syndrome, relative to age-matched comparison subjects. These findings may suggest a dysfunction in developmentally appropriate synaptic pruning, which has been described in fragile X syndrome
(35,
36). At this point, however, speculation about the mechanisms involved in larger hippocampal volumes should be tempered by the apparently contradictory findings, or lack of findings, for the structure in autism.
Because the adults with autistic disorder and the comparison subjects were not well matched on IQ, one might speculate that the differences observed in the adults with autistic disorder covary with IQ rather than diagnosis. In our opinion, two factors weigh against this interpretation. First, although IQ was not strictly matched between groups, there were no significant correlations between IQ variables and the volumetric measures. Second, since there have been previous reports of positive correlations between IQ and hippocampal volume, one would expect that the finding, if any, should have been the reverse (i.e., smaller hippocampi in the adults with autistic disorder, relative to the comparison subjects, who had higher IQs). Finally, it is worth noting with respect to IQ that previous population-based studies have indicated a higher mean IQ level in the Denver metropolitan region than in the national standardization sample for the Wechsler scales
(37), most likely due to the higher education level in the region, compared to national demographics
(38).
To our knowledge, there is only one previous study suggesting larger hippocampal volume in autism
(13) (however, see
Table 1, footnote “b”). As previously discussed, there are also negative findings with respect to hippocampal volumes in autism
(7,
8), one report of smaller hippocampal volume
(11), and one report of smaller subregional volume within the hippocampus proper
(12). In our view, the hippocampal segmentation criteria used in the current study are most similar to those used in the studies by Aylward et al.
(11) and Sparks et al.
(13). This similarity leads us to speculate that differences between the studies may be due to differences in the subject populations. A very broad range of functioning among the subjects with autistic disorder was typical in the studies, and one of the two studies reporting smaller volume had the highest functioning participants among the studies
(11), so it seems unlikely that IQ could explain the differences. Seizure history was apparently not a factor in the two studies that examined participants with autistic disorder with and without seizure disorders
(7,
12). The subjects with autistic disorder in all of the studies reporting negative findings or smaller hippocampal volume had a broader age range of participants with autistic disorder, including children and adults
(7–
9,
11,
12), than the one study reporting possible larger volume
(13), which had the most restricted age range of the studies and included only young children. The current study, however, reports larger hippocampi in an autistic disorder group entirely composed of adults. Sparks et al.
(13) have commented on the possibility that the inclusion of both child and adult autistic disorder participants, who are at different stages of hippocampal development and may have different pathological processes contributing to that development, contributes to these differing findings. Finally, while statistical power for the analysis comparing autistic disorder subjects and comparison subjects was relatively good (e.g., Cohen’s d=1.35 for the left hippocampus), the analysis comparing the parents and the comparison subjects yielded a more moderate effect (d=0.55 for the left hippocampus). It is therefore possible that the differences between this study and those listed in
Table 1 might also be due to chance.
In the present study, we found smaller left amygdala volume in adults with autistic disorder, relative to parents of children with autistic disorder and the comparison subjects, only in analyses that were adjusted for both total brain volume and gender. Adjustment for gender was particularly necessary in this comparison because of the failure to gender-match the adults with autistic disorder to the other two groups. Two previous studies have reported smaller amygdala volume in autism
(11,
17), but Haznedar et al.
(10) reported no differences in subjects with autism, and three studies have reported larger amygdala volume in autism
(9,
13,
19). Although it is clear that the subject populations and morphometric criteria differ among these studies, in our view no clear pattern emerges to explain the discrepancies among the studies. Statistical power, however, for the studies listed in
Table 1 and for the current study is low to moderate, which suggests that much larger groups of subjects may be needed to conclusively answer this question.
The ambiguity surrounding hippocampal volume in autism has implications for the interpretation of the finding of larger hippocampal volumes in the parent group in this study. Assuming that the true direction of volumetric difference is toward larger volumes, the current findings might be interpreted as evidence in support of familiality and possibly a potential genetic basis for hippocampal pathology. If, however, there is no volume difference or smaller volumes in probands, then a genetic explanation seems less likely. For the amygdala findings, since there was no suggestion of a difference between the comparison subjects and the parents of children with autistic disorder, it is less likely that differences in this structure are familial. It should be noted that studies of unaffected parents cannot discriminate genetic from nongenetic familiality—twin studies would be necessary to make this differentiation.
In light of several published papers suggesting larger brain volumes in autism (e.g., references
39–
42, but see also reference
43 for negative findings), it is worth commenting on our lack of findings with respect to total brain volume in this study. Recently, Aylward et al.
(44) reported evidence that brain size findings in autism are age-dependent, with autistic children showing larger than normal brains and adolescents and adults showing no difference in brain size, relative to age-matched comparison subjects. The authors suggested that autism is characterized by early brain overgrowth, consistent with the findings of Courchesne et al.
(41), but that there is a later decrease in brain size for people with autistic disorder at the same time that normal increases in size are seen in typically developing individuals. Thus, in adults with autism, if Aylward et al.
(44) are correct, one should not see differences in brain volume, relative to age-matched comparison subjects, which is consistent with the findings of this study.
We believe that this study is the first to report hippocampus and amygdala volumes in parents of autistic disorder probands. This approach has an advantage in that the issues of disorder severity, course, and treatment are simplified by studying clinically unaffected individuals. However, the interpretation of results from studies that include unaffected relatives will remain confusing until a clearer picture emerges concerning the nature of the deficit, if any, in affected individuals. The possibility for age-specific changes in hippocampal and amygdala volume in autism, similar to those described for total brain volume, should be explored in future studies.