Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed antidepressant medications. These medications are defined by their selective affinity for the serotonin (5-HT) transporter (5-HTT). Despite the widespread use of SSRIs, the proportion of 5-HTT sites blocked at the minimum clinical dose has been investigated for only a couple of SSRIs
(1,
2). Furthermore, the relationship between the proportion of 5-HTT sites blocked and dose (or plasma level) is unclear. This question as to the proportion of 5-HTT sites blocked at different doses of SSRIs is important: traditional assessments of SSRI effect in the clinical setting are based on treatment response, and this approach has had limited ability to distinguish differences between SSRIs or even different doses of SSRIs
(3,
4). Given that the affinities of SSRIs for the 5-HTT vary by two orders of magnitude
(5,
6), it may be that not all SSRIs block the same proportion of 5-HTT sites within the dosing range well tolerated by humans.
One barrier to assessing SSRI equivalency and dose-response relationships is the measure of clinical response itself. There is considerable variability in the likelihood of clinical response among individual subjects, and there is considerable variability in the likelihood of response across different recruiting centers for clinical trials
(3,
4).
Another factor that may contribute to difficulties in establishing a relationship between the plasma level (or dose) of an SSRI and response is that most analyses are focused on testing for a linear correlation between plasma level (or dose) and clinical response. Such a correlation is unlikely because the relationship between the plasma level of an SSRI and the proportion of 5-HTT sites blocked by the drug in the brain should be nonlinear: positron emission tomography (PET) imaging studies of other CNS medications describe a nonlinear relationship between plasma level and proportion of blockade. For example, as plasma levels of dopamine 2 (D
2) antagonist medications increase, D
2 blockade increases, and this effect also plateaus as plasma levels increase
(7,
8).
With receptor-ligand imaging, SSRI effects may now be measured on the basis of the proportion of 5-HTT sites blocked in the brain, rather than by extrapolations from clinical response or plasma concentration. [
11C]
N,N-Dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine ([
11C]DASB) PET represents a significant advance in the imaging of 5-HTT sites
(2,
9–12). [
11C]DASB is highly selective, showing nanomolar affinity for the 5-HTT and negligible affinity for other receptors
(9,
12). A commercial screen (Novascreen Biosciences Corp., Hanover, Md.) for DASB affinity at 41 receptor, enzyme, and ion-channel assays, using a concentration of 1000 nM, showed that DASB was inactive at all sites except for the 5-HTT, where greater than 95% inhibition of binding was found
(10). The main advantage of [
11C]DASB over [
11C](+)McN5652, another PET radioligand, is that [
11C]DASB has a much higher ratio of specific binding to nonspecific binding in vivo. As a result, the binding potential values found with [
11C]DASB PET are approximately two- to threefold greater than those found with [
11C](+)McN5652 PET
(9,
11–13). (Binding potential is proportional to receptor density.) Furthermore, the 5-HTT binding potential found with [
11C]DASB PET is reliably found in the test-retest condition for most brain regions
(2). With [
11C]DASB PET, 5-HTT binding potential values are detectable in the frontal cortex and are reasonably high in the basal ganglia and thalamus, even without arterial sampling
(11,
12). With [
11C](+)McN5652 PET, 5-HTT binding potential values are modestly detectable in the thalamus
(14) and probably the basal ganglia, although this may require concurrent arterial sampling of the radiotracer
(15). [
123I]2-Beta-carbomethoxy-3-beta-(4-iodophenyl)-tropane ([
123I]β-CIT) is a radiotracer used in single photon emission computed tomography (SPECT), and it was the first radioligand available for imaging of 5-HTT sites. [
123I]β-CIT is much less selective for the 5-HTT than is [
11C]DASB
(9,
10,
16,
17). To our knowledge, the test-retest reliability for the 5-HTT binding potential found with [
123I]β-CIT SPECT or [
11C](+)McN5652 PET has not been published.
There have been a few investigations of 5-HTT occupancy after treatment with SSRIs. Occupancy is the percent reduction in binding potential after drug administration and may represent the proportion of receptor sites blocked by medication. One study using [
123I]β-CIT SPECT showed that depressed subjects treated with 20–60 mg/day of citalopram had a 50% lower 5-HTT binding potential in the combined thalamus and brainstem regions than did healthy subjects
(1). A case report of a single subject by the same group using [
123I]β-CIT SPECT indicated that after treatment with fluoxetine 5-HTT binding potential was 40% lower than that in a pool of healthy subjects
(18). These measures are thought to substantially underestimate 5-HTT occupancy because [
123I]β-CIT also binds with high affinity to dopamine transporter sites
(16,
17).
We previously reported that there was an 80% reduction in the 5-HTT binding potential after 4 weeks of treatment with either citalopram or paroxetine at 20 mg/day
(2). In our study
(2), 5-HTT binding potential was measured before and after treatment in a within-subject design by using [
11C]DASB PET. To our knowledge, the occupancy for other SSRIs at minimum treating doses (as determined with a radiotracer selective for the 5-HTT) is unknown at this time.
The first purpose of the present study was to determine 5-HTT occupancy at minimum therapeutic doses for three additional SSRIs (fluoxetine, sertraline, venlafaxine). The second purpose was to determine the 5-HTT occupancy during treatment with five SSRIs at a wide range of doses well tolerated by humans, by using [
11C]DASB PET and a within-subject design. SSRIs were defined as having affinities for the 5-HTT at least two orders of magnitude greater than that for other receptors. On this basis, the SSRIs chosen were citalopram, fluoxetine, sertraline, paroxetine, and venlafaxine
(5,
6).
Method
Subjects
This study was approved by the University of Toronto Human Subjects Review Committee. Eighty-four subjects were recruited, and 77 subjects completed the entire protocol. Subjects who completed the protocol were 20 to 50 years old (33 female and 44 male; mean age=35 years, SD=9). Twelve of these 77 subjects were described in a previous report
(2). The groupings were designed so as to treat patients with therapeutic doses and to avoid giving healthy subjects therapeutic doses for 4 weeks. (The antidepressants are proven therapeutic agents, and it was unnecessary to administer them to healthy subjects; see the following.) This led to three dosing groups. The first group (N=37) were primarily healthy subjects, and they received no more than half of the minimum typical daily therapeutic dose of each SSRI (citalopram: 1–10 mg; fluoxetine: 1–10 mg; sertraline: 5–25 mg; paroxetine: 5–10 mg; extended-release venlafaxine: 2.4–37.5 mg). The second group (N=29) had a major depressive episode secondary to major depressive disorder, and they received usual treating daily doses of SSRIs (citalopram: 20–40 mg; fluoxetine: 20 mg; sertraline: 50–100 mg; paroxetine: 20 mg; extended-release venlafaxine: 75 mg). The third group (N=16) had major depressive disorder and a current comorbid anxiety disorder, an anxiety disorder (obsessive-compulsive or panic disorder), or treatment-resistant major depressive disorder. They received daily doses of SSRIs typically used to treat such illness (citalopram: 40–60 mg; fluoxetine: 40–60 mg; sertraline: 150–200 mg; paroxetine: 40–60 mg; extended-release venlafaxine: 150–225 mg). Two subjects in the second group experienced significant side effects after a few days, were moved into the first group, and received very low doses of antidepressants for 4 weeks (thus, 35 of the 37 subjects in the first group were healthy). Five subjects completed the protocol in the second group, were poorly responsive to treatment, and reenrolled in the protocol to join the third group; they are therefore included in both the second and third groups. The subjects did not take any other medications during SSRI treatment. Overall, the combined ranges covered subtherapeutic dosing as well as a full range of clinically relevant doses. Our previous investigation
(2) and preliminary findings showed that the baseline values for striatal 5-HTT binding potential were similar across these patient groups; hence, biases in occupancy from using both healthy subjects and patient groups would be negligible.
For each subject, written consent was obtained after the procedures had been fully explained. The healthy subjects were screened by using the Structured Clinical Interview for DSM-IV (SCID), nonpatient version
(19). For patients, the diagnosis was confirmed by the patient version of the SCID
(20), which was administered by a trained research assistant (S.S.). Each patient also received a psychiatric consultation (by J.H.M. or A. Cheok) to verify the SCID diagnosis. Patients with psychotic symptoms or bipolar disorder (type I or II) were excluded, as were subjects with a history of alcohol or drug abuse or dependence. In addition, all past drug use was recorded. No subject had a history of exposure to 3,4-methylenedioxymethamphetamine or other drugs suspected to have neurotoxic effects on neurons expressing 5-HTT
(21). There were seven patients with a history of substance use (six subjects had used marijuana, one subject had used an opiate) that did not meet the criteria for the SCID diagnosis of substance abuse. These seven patients had not used substances within the previous 6 months. Each received a urine drug screen, the results of which were negative. All healthy subjects received a urine drug screen.
Thirty-one of the 40 patients had never completed an antidepressant trial. The remaining patients had not taken antidepressant medication within the previous 1 month (plus five half-lives of the medication) before the first scan. Each patient had routine tests to rule out common medical causes of mood and anxiety disorders (thyroid function tests, electrolyte measurements, complete blood cell count).
[11C]DASB Positron Emission Tomography Imaging
Each enrolled subject received an [11C]DASB PET scan before and after administration of an SSRI. The second scan occurred after 4 weeks of SSRI administration at the maximum dose allotted for the subject.
Before treatment, the subjects were informed that their serum SSRI levels would be sampled on the day of the second [
11C]DASB PET scan. The serum sampling and second [
11C]DASB PET scan took place 6 to 13 hours after the last dose. The blood samples taken were frozen at –20°C and subsequently assayed by using high-performance liquid chromatography with fluorescence detection (Medical Toxicology Unit, Guy’s and St. Thomas’ Hospital Trust, London)
(22).
The synthesis of [
11C]DASB has been described previously
(9,
10). Just before the PET scan, an intravenous bolus of 370 MBq of [
11C]DASB was injected. The [
11C]DASB was of high radiochemical purity (>95%) and high specific activity (mean=32 GBq/μmol, SD=10, at the time of injection). PET images were obtained by using a GEMS 2048-15B camera (Scanditronix
Medical, Uppsala, Sweden) (intrinsic resolution full width at half maximum=5.5 mm). Images were obtained in 15 1-minute frames, followed by 15 5-minute frames. The images were corrected for attenuation by using a
68Ge transmission scan and were reconstructed by filtered back projection (ramp filter with a Hann window).
Data Analysis
To obtain a measure of the 5-HTT binding potential with region-of-interest data, we used the noninvasive method of Logan et al.
(23) implemented within a standard software package
(24). This model assumes that there is a region of interest that contains specifically bound radioligand and that there is a reference region that does not contain specifically bound radioligand. For [
11C]DASB, the cerebellum is a suitable reference region because studies have shown either undetectable
(25) or extremely low
(26,
27) 5-HTT density. More recently, it was found with the Western blot method that the 5-HTT density in the cerebellum is less than 3% of the striatal value (S. Kish et al., personal communication, 2003). The model also assumes that the concentration of radiotracer in the cerebellum divided by the concentration of radiotracer in the region of interest becomes constant. In addition, the radiotracer must be sufficiently reversible such that the plot becomes linear. In our data sets, these assumptions are met sufficiently early and the plots were linear between 15 and 65 minutes in all brain regions except the midbrain, for which the plots were linear before 70 minutes. Another important assumption is that the free and nonspecific uptake of [
11C]DASB is reasonably similar among subjects, and this was shown by fairly similar cerebellum distribution volumes among the subjects in our earlier data set (the coefficient of variation, i.e., standard deviation/mean, for the distribution volume in the cerebellum was 13%).
Previous work at our center has demonstrated that the 5-HTT binding potential found by using methods based on reference tissue is highly correlated with the ratio of the kinetically determined distribution volumes in regions with specific binding to the distribution volume in a reference region
(12). In this data set
(12), we measured the distribution volumes in the thalamus, striatum, frontal cortex, and cerebellum in a set of healthy subjects by using an arterial input function and a single tissue compartment model. For regions with specific binding, the ratio of the distribution volume in each region to the distribution volume in the cerebellum was found, and this ratio was highly correlated with the binding potential found with the noninvasive method of Logan et al.
(23) (r=0.98, N=15, p<0.001).
The bilateral striatum was chosen as the primary region with specific binding because [
11C]DASB uptake is high and homogeneous in the striatum
(11), absolute test-retest differences in 5-HTT binding potential for this region in preliminary analyses were low (approximately 10%)
(2), and we know of no reports that major depressive disorder itself influences 5-HTT receptor density or affinity in this region. The thalamus is another large brain structure with high 5-HTT density (albeit somewhat less homogeneous) and similar test-retest differences in 5-HTT binding potential with [
11C]DASB PET. The binding potential is proportional to B
max/K
d (28). (For further explanation, see reference
29.) B
max represents receptor density. K
d is the dissociation constant and is inversely proportional to affinity.
Each subject had a magnetic resonance imaging (MRI) scan (GE Signa 1.5-T scanner, spin-echo sequence proton density weighted image; x, y, z voxel dimensions 0.78, 0.78, and 3.00 mm, respectively). For the primary analysis of regions of interest, the whole striatum and cerebellum regions of interest were drawn on summated [
11C]DASB PET scans with reference to a coregistered MRI
(30) by a rater blinded to the identity of the scans (A. Carella). Occupancy was also measured in secondary regions: prefrontal cortex (Brodmann’s areas 8, 9, 10, and 46), anterior cingulate (Brodmann’s areas 32 and 24), bilateral thalamus (two contiguous planes), bilateral cuneus (three contiguous planes), and midbrain (two contiguous planes). The method of determining regions of interest in the striatum, thalamus, prefrontal cortex, anterior cingulate, and cerebellum to measure occupancy has been described previously
(2,
7,
31,
32). For the midbrain region of interest, the border of the midbrain was drawn in the two planes superior to the pons. In two subjects, only one plane of the midbrain was used, as the pons and midbrain structures were smaller in these two subjects. The 5-HTT binding potential found in these regions was reasonably reliable in the healthy subjects who had undergone test-retest measurements, i.e., the mean absolute difference in the repeated measures of 5-HTT binding potential was less than 15% of regional 5-HTT binding potential with the exception of the cuneus, for which the mean absolute difference in test-retest measurements was less than 20% of the 5-HTT binding potential.
Occupancy in regions with specific binding was defined as the between-scan difference in 5-HTT binding potential (scan 1 minus scan 2) divided by the 5-HTT binding potential at scan 1. Occupancy expressed as a percentage was calculated for each subject. For the five subjects who received a third [11C]DASB PET scan at a higher dose of SSRI, the occupancy at the higher dose was based on the 5-HTT binding potential at scan 1 (drug-free state) and scan 3 rather than scan 1 and scan 2, i.e., occupancy was defined as the difference in 5-HTT binding potential between scans 1 and 3 divided by the 5-HTT binding potential at scan 1.
Results
Baseline Striatal 5-HTT Binding Potential
The subjects were divided into the following diagnostic categories in order to assess baseline differences within this study group: healthy (N=35), major depressive episode secondary to major depressive disorder (N=37), panic disorder (N=7), and obsessive-compulsive disorder (N=7). Some subjects had more than one diagnosis and were assigned to more than one group. The baseline striatal binding potentials for these subgroups were 1.0 (SD=0.2), 1.0 (SD=0.2), 1.0 (SD=0.2), and 1.0 (SD=0.2), respectively. There were no significant differences between the patient subgroups and healthy subjects according to analysis of variance (ANOVA) (depressive episode: F=0.29, df=1, 70, p=0.59; panic disorder: F=0.31, df=1, 40, p=0.58; obsessive-compulsive disorder: F=0.13, df=1, 40, p=0.72). A second analysis was done to compare subjects with major depressive episodes and no comorbid diagnoses (N=29) to healthy subjects, and no significant differences in baseline striatal binding potential were found; the mean baseline striatal binding potential of the depressed patients without comorbid illnesses was 1.0 (SD=0.2) (F=0.14, df=1, 62, p=0.71).
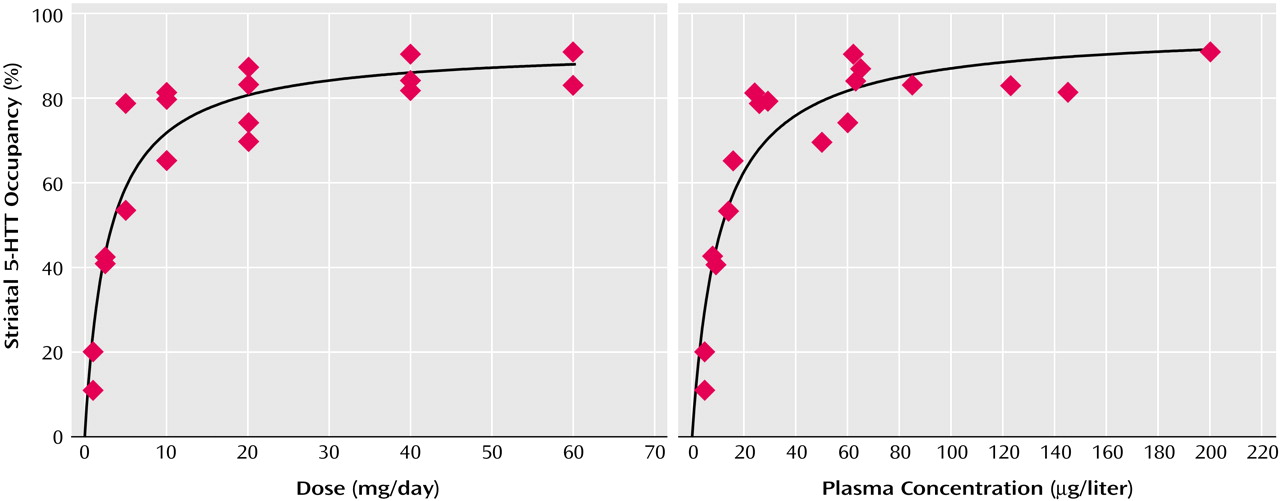
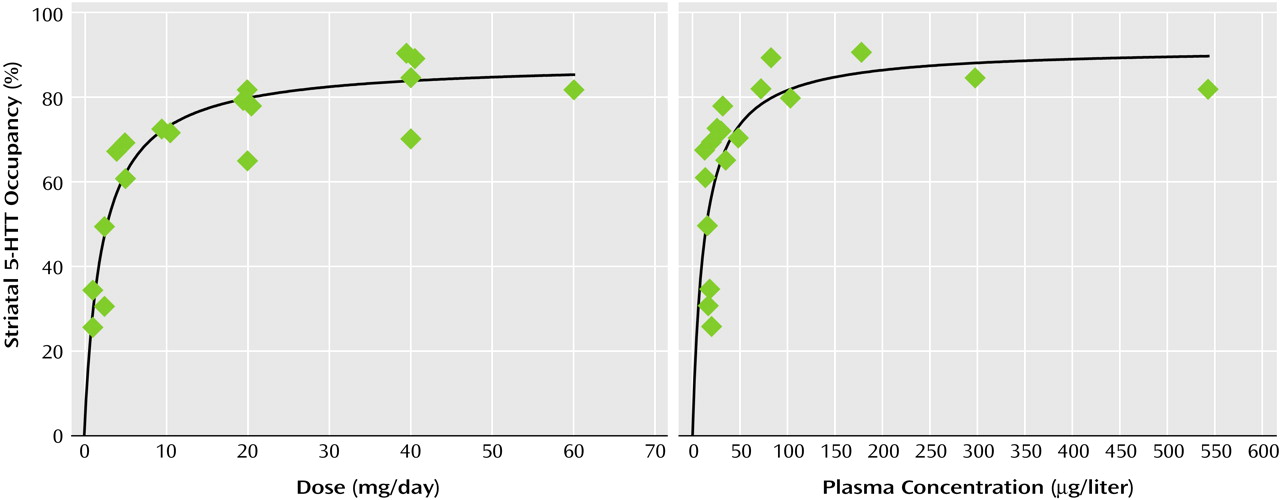
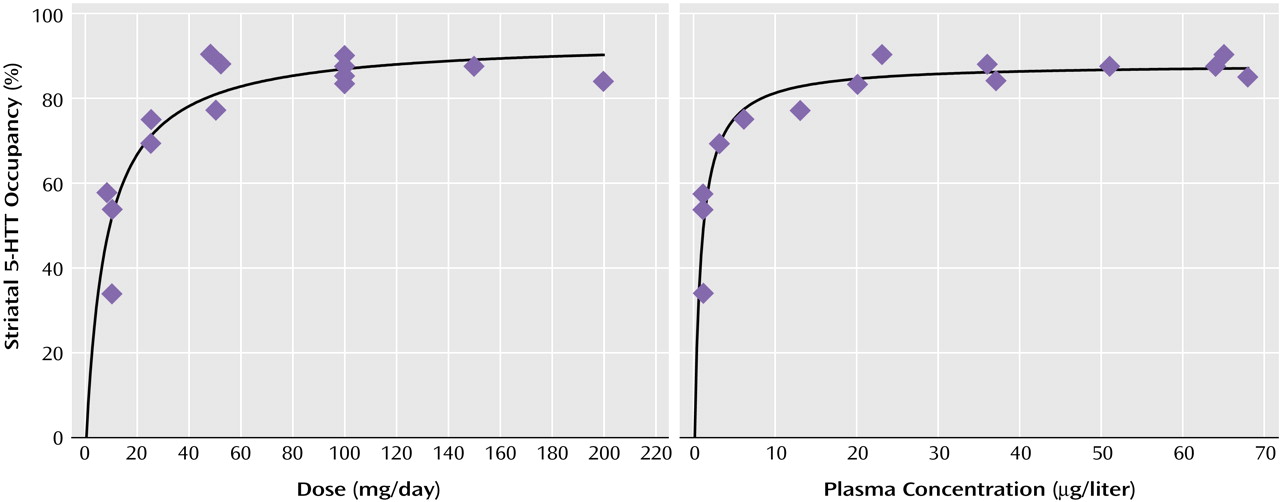
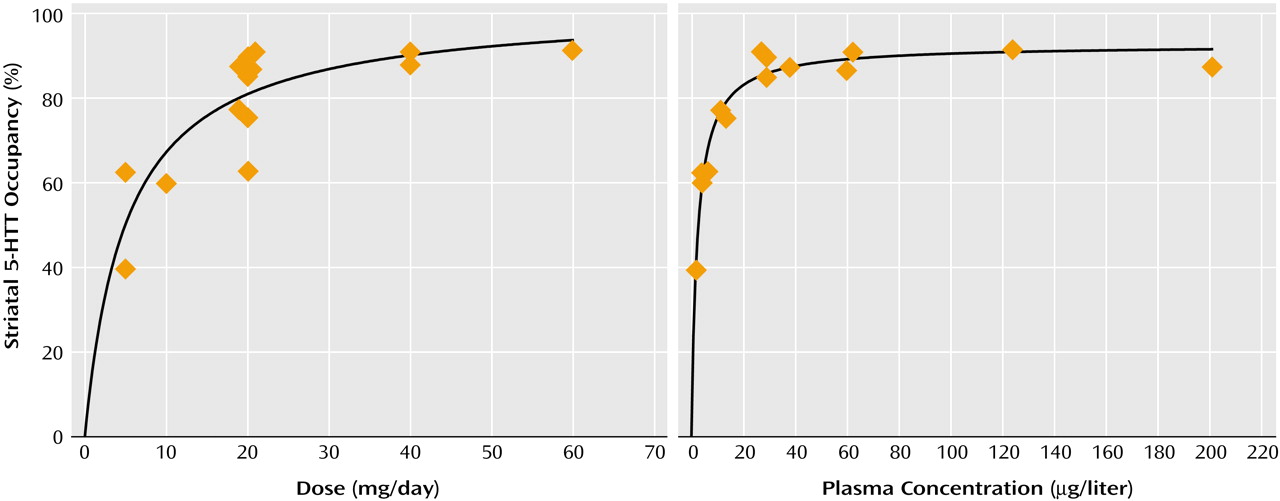
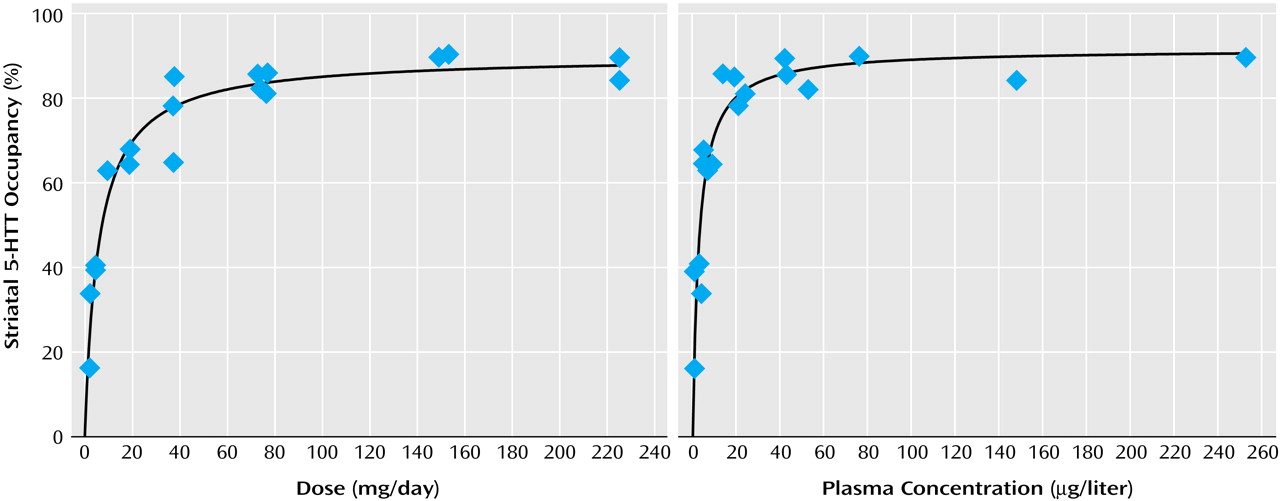
Relation of Striatal 5-HTT Occupancy to SSRI Dose or Plasma Concentration
For each drug, substantial occupancy occurred at subclinical doses (
Figure 1,
Figure 2,
Figure 3,
Figure 4, and
Figure 5). Occupancy increased as the dose or plasma level increased, with plateauing at higher doses and plasma levels. A standard function in the field of pharmacology was used. The function was f(x)=a(x/[b+x]), where
f(
x) refers to occupancy and
x refers to dose or plasma level of the SSRI. This function significantly fit the data.
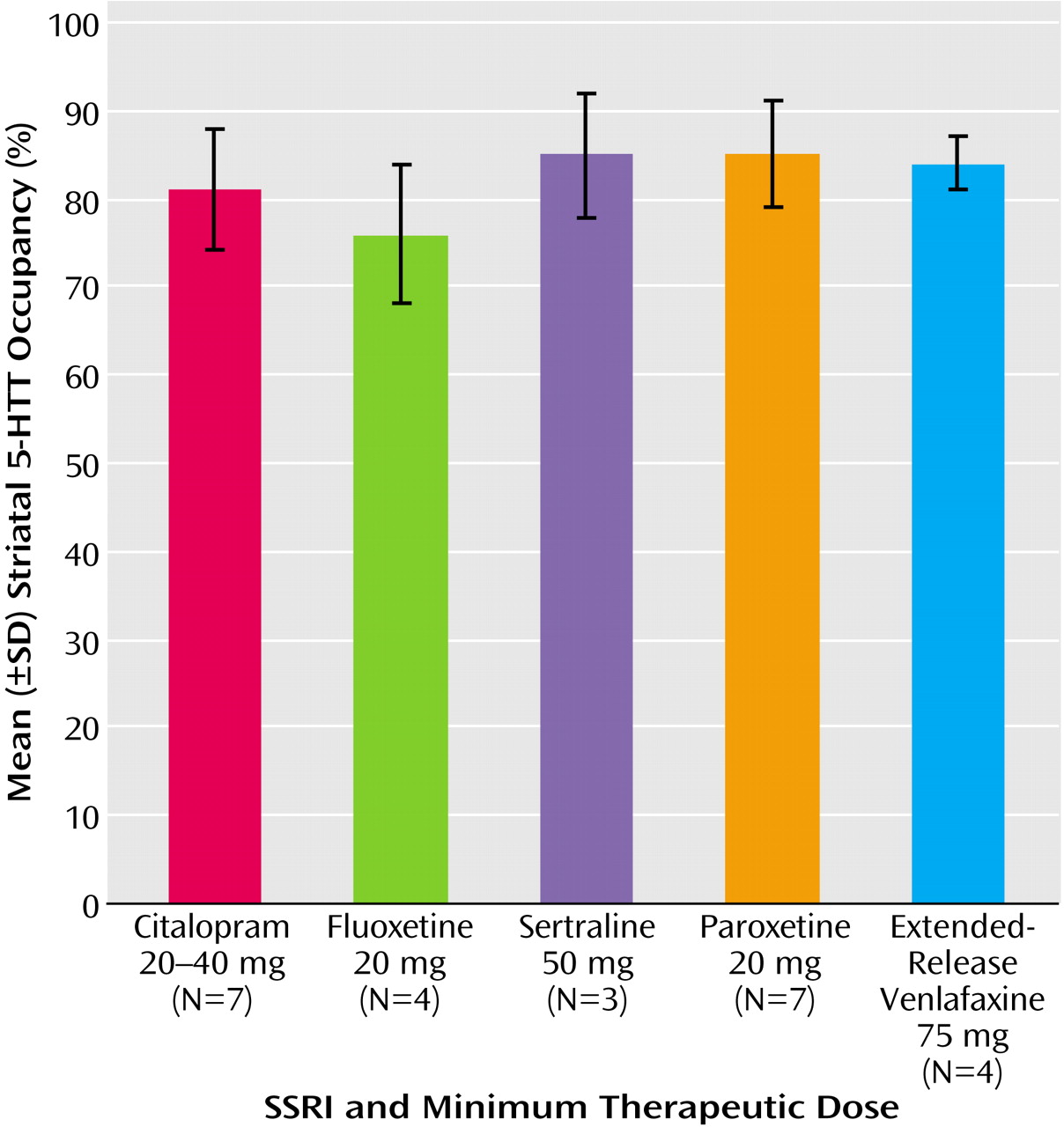
For all drugs, approximately 80% occupancy occurred at doses that are significantly better than placebo in the treatment of depressive episodes (
Figure 6)
(4,
33–36). For each SSRI the minimum recommended daily treating dose for major depressive episode resulted in a decline in striatal 5-HTT binding potential that was significantly different from our test-retest measurements of striatal 5-HTT binding potential previously reported for six healthy subjects
(2), according to repeated-measures ANOVA (citalopram, 20–40 mg: F=84.68, df=1, 11, p<0.001; fluoxetine, 20 mg: F=73.03, df=1, 8, p<0.001; paroxetine, 20 mg: F=91.61, df=1, 12, p<0.001; sertraline, 50 mg: F=116.20, df=1, 7, p<0.001; extended-release venlafaxine, 75 mg: F=126.92, df=1, 8, p<0.001).
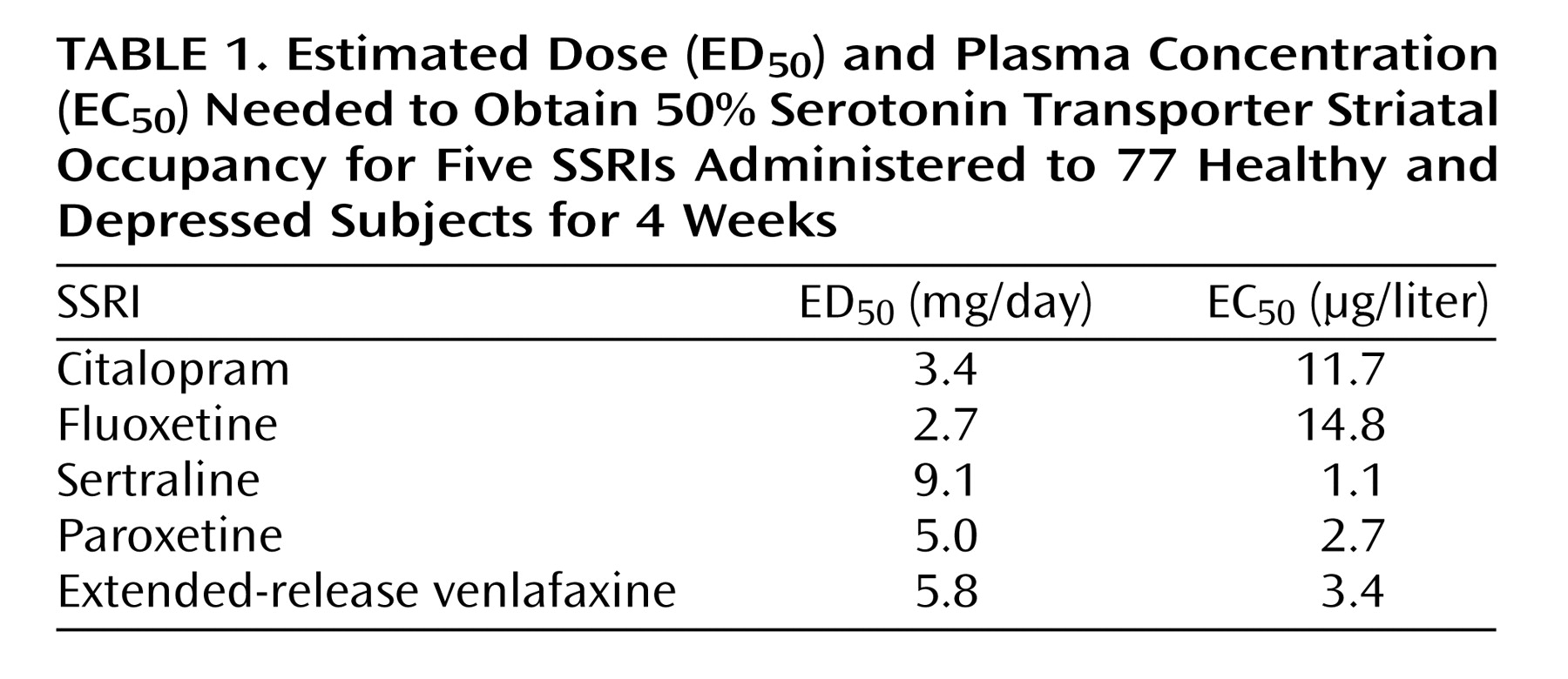
The estimated dose and plasma concentration needed to obtain a 5-HTT occupancy of 50% (ED
50 and EC
50, respectively) were calculated for each SSRI (
Table 1). To determine whether these values could be predicted by SSRI affinity alone, we compared these values to reported affinities (K
d) for the cloned human 5-HTT
(6). The K
d values (in nanomoles) reported by Tatsumi et al.
(6) were 1.16, 0.81, 0.29, 0.13, and 8.9 for citalopram, fluoxetine, sertraline, paroxetine, and venlafaxine, respectively. There was no significant linear correlation between ED
50 and affinity (r=0.30, N=5, p=0.63). There was no significant linear correlation between EC
50 and reported affinity (r=–0.22, N=5, p=0.73). There was also no correlation between log-transformed values for affinity for the 5-HTT (pK
d) and ED
50 (r=0.04, N=5, p=0.96) or EC
50 (r=0.18, N=5, p=0.78).
Relation of Striatal 5-HTT Occupancy to Clinical Response
While the number of subjects who completed the protocol was large for a PET imaging study, it was small from the perspective of a clinical trial. Even so, we also examined the relationship between clinical response, measured with the 17-item Hamilton Depression Rating Scale
(37), and the striatal occupancy. In 36 subjects who had a diagnosis of major depressive episode, there was no relationship between striatal occupancy and percent change in Hamilton depression scale score from scan 1 to scan 2 (r=0.20, N=36, p=0.24). There was also no relationship between striatal occupancy and the presence at scan 2 of a Hamilton depression scale score reflecting clinical response (less than 8) (independent samples t test comparing 17 nonresponders or partial responders and 19 responders: t=0.08, df=34, p=0.94).
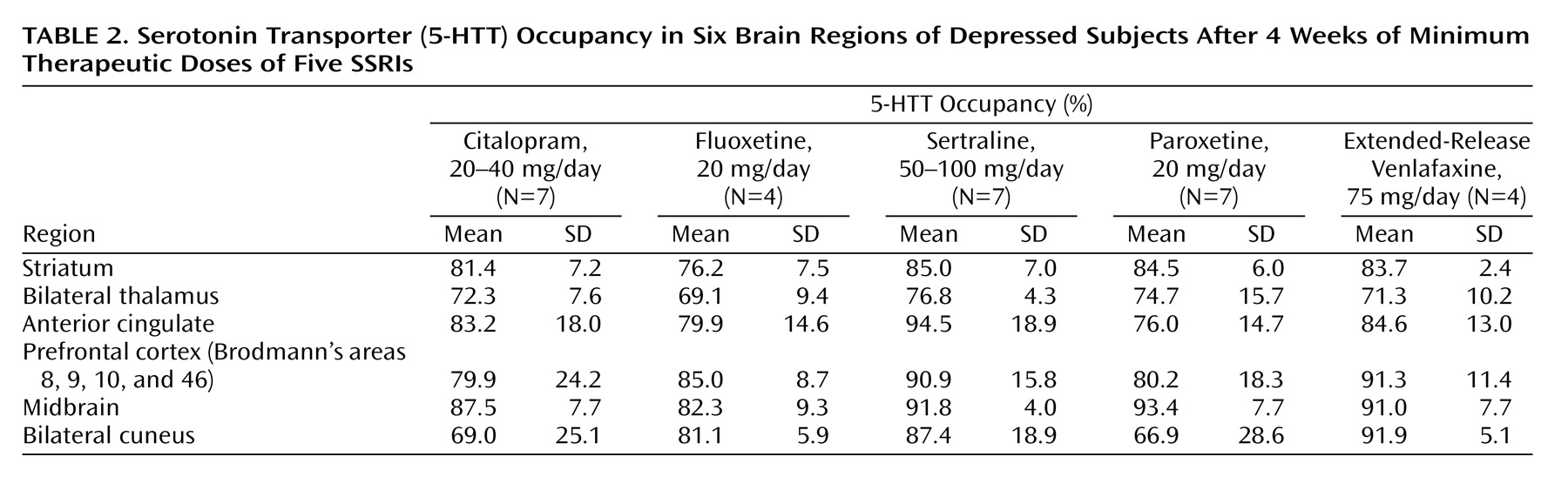
5-HTT Occupancy in Other Regions
While our primary region of interest for 5-HTT occupancy was the striatum, we also measured it in other regions of subjects who had received the minimum recommended treating dose for major depressive episode. The occupancies in these other regions are listed in
Table 2. These occupancies tended to be correlated with the striatal occupancy (N=25, r=0.36 to r=0.73, p=0.08 to p<0.001). Post hoc analyses showed that in comparison to the striatal 5-HTT occupancy, the 5-HTT occupancy in the thalamus was significantly lower (8% lower) (paired t test: t=–4.35, df=24, p<0.001) and that the 5-HTT occupancy was significantly higher in the midbrain (8% higher) (paired t test: t=6.53, df=24, p<0.001). The average 5-HTT occupancies in the other regions (prefrontal cortex, anterior cingulate, cuneus) were similar to the average striatal 5-HTT occupancy. The mean 5-HTT occupancies in these other regions were within 3% of the mean striatal 5-HTT occupancy.
Discussion
In this study we found that in our primary region of interest, the striatum, the 5-HTT occupancy at minimum therapeutic doses of five different SSRIs was approximately 80%. It was also observed that 5-HTT occupancy increased as plasma level and dose increased and that 5-HTT occupancy plateaued at high plasma levels or doses.
It is interesting that the daily doses of SSRIs that are convincingly distinguishable from placebo in the clinical setting—20 to 40 mg for citalopram, 20 mg for fluoxetine, 50 mg for sertraline, 20 mg for paroxetine, and 75 mg for extended-release venlafaxine
(4,
33–36)—were also the doses that obtained an 80% occupancy in the striatum. The occupancy data indicate that with these doses, the blockade at the 5-HTT is fairly equivalent across SSRIs. It also suggests that an 80% occupancy of the 5-HTT is a necessary minimum for SSRI treatment of depressive episodes.
The consistency of an 80% striatal occupancy at minimum therapeutic doses may appear surprising because the SSRIs chosen had a wide spectrum of affinity for the 5-HTT, covering two orders of magnitude (K
i ranging from about 0.1 nM for paroxetine to 10 nM for venlafaxine)
(5,
6). Therefore, 5-HTT occupancy is influenced by factors besides affinity. The amount of drug given and its plasma level have an obvious role, demonstrated by the nonlinear correlations between plasma concentration (or dose) and occupancy. The low, nonsignificant correlations of reported affinity to ED
50 and EC
50 suggest that affinity cannot predict the dose (or plasma level) that will achieve a 50% occupancy among high-affinity medications. This suggests that an important pharmacological property besides affinity is the ability of the SSRI to penetrate the brain and accumulate near the synapse and axons, where 5-HTT are found
(38).
Given that there are pharmacological properties besides affinity that influence occupancy, it may be presumptuous to assume substantial occupancy on the basis of affinity alone. Many of the SSRIs included in this study have some affinity for other monoamine transporter sites. For example, venlafaxine and paroxetine have an affinity for the norepinephrine transporter site that is two orders of magnitude less than their affinity for the 5-HTT
(5,
6). Sertraline has an affinity for the dopamine transporter site that is two orders of magnitude less than its affinity for the 5-HTT
(6). Without direct occupancy measures, it is unclear whether these medications have substantial occupancy at other monoamine sites.
The ED
50 occurred at very low doses, and the dose-occupancy curves reveal substantial striatal 5-HTT occupancy at low doses of SSRIs. This information may be useful for the clinician faced with a patient who does not tolerate the minimum therapeutic dose. The 80% occupancy need not be obtained with a single SSRI. For rare patients who cannot tolerate the minimum doses that distinguish SSRIs from placebo, the clinician could consider combinations of two SSRIs at doses that individually achieve 60%–70% striatal occupancy in order to achieve 80% occupancy during the initial therapeutic trial. Ideally, clinical trials of low-dose SSRI combinations should be done in order to validate this approach; however, we believe that such data are not currently available. The data of this study do not provide an argument for subtherapeutic dosing of SSRIs even though substantial occupancy may be obtained in this manner. It is conceivable that some of the proposed antidepressant mechanisms, such as increasing synaptic 5-HT concentrations
(39,
40), increasing 5-HT neurotransmission
(41), or creating neurotrophic effects
(42,
43), may occur only at 80% occupancy.
Our post hoc comparisons showed that the 5-HTT occupancy in the striatum tended to correlate with the 5-HTT occupancy in the prefrontal cortex, anterior cingulate, and cuneus and that the overall mean 5-HTT occupancies of these three regions were within 3% of the overall mean 5-HTT occupancy in the striatum. This suggests that the concentrations of SSRIs in these regions are similar. Our other post hoc comparisons of regional occupancy indicated that 5-HTT occupancy in the midbrain was significantly higher than occupancy in the striatum and that 5-HTT occupancy in the thalamus was significantly lower. This suggests that SSRI concentrations may be higher in the midbrain and lower in the thalamus. It may be that in brain regions with very high 5-HTT density (such as the raphe nuclei in the midbrain), there are higher concentrations of SSRIs. For radiotracers, it is well known that regions with higher concentrations of specific binding sites have greater concentrations of radiotracer. It may be that an analogous situation occurs with some drugs such that regions with very high densities of specific binding sites also have higher concentrations of drug that bind to these sites. It is possible that there are differences between the thalamus and striatum that result in different SSRI concentrations; for example, one may speculate that the degree of lipophilicity of the SSRI drugs results in a lower absorption of SSRIs in the thalamus. Even though the 5-HTT occupancy in two other regions was different from that in the striatum, we chose the striatum a priori as the principal region of interest: the striatum is an ideal structure for occupancy measurement because it is large, it has a homogeneous uptake of [11C]DASB, and the consistency of test-retest measurements is excellent. The actual mean absolute difference in test-retest measurements expressed as a percentage of the striatal 5-HTT binding potential for the six subjects scanned twice with [11C]DASB PET in the present study was 8%.
For some SSRIs at higher doses, striatal occupancies of greater than 80% may occur. Whether it is better to treat with SSRIs in order to achieve 5-HTT occupancies greater than 80% is currently unknown, to our knowledge. The information from this study would allow for clinical trials with SSRIs at higher occupancies so as to investigate this question. The occupancy data may also be used to make informed post hoc analyses of existing clinical trial data: doses or plasma levels corresponding to striatal occupancy values of 85% to 90% may be compared to doses or plasma levels corresponding to 80% occupancy. An 80% occupancy occurs with SSRI doses that are superior to placebo, but an 80% occupancy may not necessarily be the most therapeutic occupancy.
Occupancy measurement with [11C]DASB PET should be incorporated into antidepressant development for compounds that target the 5-HTT. New antidepressants may be selected from candidates on the basis of having an 80% or greater striatal occupancy for the 5-HTT. Dosing in humans could be optimized on the basis of occupancy data before clinical trials are conducted. For example, for antidepressants with high affinity for multiple sites, the therapeutic effect of 5-HTT blockade could be optimized by dosing to achieve 80% or greater occupancy.
Metabolites of some SSRIs have been considered to make substantial contributions to overall occupancy. The differential contribution of metabolites to overall occupancy cannot be assessed in this type of study because the concentration of the parent compound is associated with the concentration of metabolites. It is conceivable that a major metabolite may contribute substantially to occupancy and that the effect is masked by the strong association between plasma levels of the parent SSRI and major metabolites. To investigate the specific contribution of metabolites, future investigations should measure occupancy after administration of the metabolite alone.
We did not find a 100% occupancy in humans with [
11C]DASB PET. We think the reason for this is that the currently available SSRIs do not achieve 100% occupancy within typical dosing ranges at the times chosen for measuring the 5-HTT occupancy. Using [
11C]DASB methods, we have observed 92% striatal occupancy after a single dose of fluoxetine at 5 mg/kg in cats
(44) and 95% striatal occupancy after a single dose of 2 mg/kg of [
11C](+)McN5652
in rats
(9). The occupancies found in the midbrain
(44) and thalamus
(9,
44) were virtually identical to those for the striatum in these studies.
There are several limitations to this study. 5-HTT binding potential was measured 6 to 13 hours after the last SSRI dose; hence, the occupancy represents this time period. It is theoretically possible that occupancy could be lower after 13 hours, but this is unlikely because the occupancy was found after 4 weeks at the dose administered and there was no systematic change in occupancy within the 6 to 13 hours after the last dose for any SSRI. Strictly speaking, the occupancy measure represents the change in binding potential. Given that the binding potential is proportional to receptor density and affinity, occupancy may also include processes such as down-regulation
(45). Nevertheless, most 5-HTT are located perisynaptically and along axons
(38), and the 5-HTT to which [
11C]DASB can bind probably reflect the 5-HTT available for clearance of extracellular serotonin. We chose to measure occupancy in three groups of subjects, two of which had psychiatric illnesses. Since the 5-HTT occupancy is high at clinical doses and the observable difference in baseline 5-HTT binding potential between diagnostic categories is minimal in the bilateral striatum, the effect of illness on 5-HTT occupancy is negligible.
We believe that this is the first study to investigate 5-HTT occupancy of multiple SSRIs at multiple doses by using a highly reliable imaging method. We have demonstrated that SSRIs have increasing striatal occupancy with increasing plasma level or dose and that a reasonably consistent striatal occupancy of 80% occurs at doses that are superior to placebo. These results would not be predicted by affinity of the individual SSRIs for the 5-HTT alone. The finding of an 80% striatal occupancy across five SSRIs at doses that are clinically distinguishable from placebo also argues strongly for incorporating these 5-HTT occupancy measures into future antidepressant development. The other interesting finding was that some SSRIs can actually achieve 5-HTT occupancies greater than 80% in the clinical dosing range. Clinical studies of high 5-HTT occupancy treatments may provide an important therapeutic opportunity for both existing and future antidepressant medications.