Women who have suffered one episode of major depression following childbirth have a risk of recurrence of about 25%
(1). Postpartum-onset major depression provides an opportunity for prevention because its onset is preceded by a clear marker (birth), there is a defined period of risk for illness onset (3 months)
(2), and a high-risk sample of mothers (with previous episodes of major depression) is identifiable
(1). The theoretical framework of this project was the hypothesis that administration of an antidepressant to
asymptomatic women after birth would prevent the recurrence of postpartum-onset major depression.
We completed a randomized clinical trial with the selective serotonin reuptake inhibitor (SSRI) sertraline, which was selected because data were available to support its use by breast-feeding women
(2). The hypotheses were 1) the rate of recurrence of postpartum-onset major depression would be lower in the women treated with sertraline than in those who received placebo and 2) the time to recurrence would be longer for the sertraline-treated women than for those given placebo.
Method
The subjects were pregnant with gestations of 35 weeks or less, age 21–45 years, and healthy with normal results from thyroid studies and a complete blood count. Each woman had had at least one episode of postpartum-onset major depression that fit the DSM-IV criteria for major depression
(3) within 5 years of enrollment. The subjects were not depressed during the index pregnancy. Women who chose to continue psychotherapy or use psychotropic medications after the first trimester were ineligible. Women who met the criteria for any other axis I diagnosis (except generalized anxiety or panic disorder) or for antisocial or borderline personality disorder and those who had psychosis or bipolar disorder were excluded. Approval was obtained from the institutional review board, and written informed consent was obtained from all subjects.
The pregnant women were evaluated with the Hamilton Depression Rating Scale
(4) and the Structured Clinical Interview for DSM-IV
(3) in a women’s psychiatric outpatient program. A second interview was scheduled between 32 and 36 weeks to ensure that the subject did not develop major depression during pregnancy. The subjects were assigned randomly in a 2:1 (sertraline:placebo) ratio because our previous randomized clinical trial that compared nortriptyline to placebo
(1) revealed no drug effect, and we wanted to determine rapidly whether this was also the case for sertraline. The blind was continued until all subjects completed the protocol.
The randomized clinical trial phase began immediately postpartum. The study drug was delivered to the subject in the maternity hospital to achieve dosing as soon as possible after birth. Thereafter, the sertraline or placebo was given as a single postbreakfast dose in two identical opaque gelatin capsules.
We began the dosing protocol with 50 mg/day. However, the first two subjects withdrew because of severe headaches. A dose reduction to 25 mg/day for 4 days was recommended by the nonblind monitoring team. Thereafter, the dose was increased to 50 mg/day through week 4, then to 75 mg/day during weeks 5–17. At study week 17 the dose was tapered across 3 weeks, and treatment was discontinued at week 20. Serum levels were determined at weeks 2, 3, 4, 6, 8, 11, 14, and 17 to assess compliance. Side effects were recorded with the 25-item Åsberg Side Effects rating scale
(5).
If the subject had a Hamilton depression scale score of 15 or higher on two occasions 1 week apart, she was evaluated by a blinded psychiatrist (K.L.W., C.M.P.) to confirm the presence of DSM-IV criteria for major depression. Because the postpartum period also is associated with first episodes of hypomania or mania
(2), the mania rating scale from the Schedule for Affective Disorders and Schizophrenia
(6) was given weekly. A score of 12 or higher triggered an evaluation by a psychiatrist.
Results
We screened 38 eligible women; 25 consented to participate. Three women who were randomly assigned to sertraline never took it; therefore, the data are derived from 22 subjects (sertraline, N=14; placebo, N=8). The mean age of the subjects was 32 years (SD=3, range=25–37), with no difference between the women taking sertraline and placebo (t=0.34, df=20, p=0.60). All of the women were white, married, and of middle to high socioeconomic status.
The average time from birth to first dose was 15.6 hours (SD=9.6, range=2.5–48.5), with no difference between mean time to receipt of the drug between groups (t=0.92, df=20, p=0.37); for placebo it was 18.1 hours (SD=14.3), and for sertraline it was 14.2 hours (SD=5.6). The women in the study were compliant, as evidenced by ranges of maternal serum sertraline and N-desmethylsertraline levels of 23–48 and 36–66 ng/ml, respectively, across samples from the 8 weeks of collection. None of the personnel or subjects was more successful than chance at identifying the drug assignment. The rates of agreement with the assigned condition ranged from 38% (for the blinded mood assessors) to 71% (for the side effects monitors), which did not differ significantly from the expected 44%–57% (Fisher’s exact test, p=0.14–0.53).
There was no difference in the number of women who withdrew from the study (p=0.35, Fisher’s exact test) or in the time to withdrawal (p=0.85, Freeman-Halton extension of Fisher’s exact test) between subjects in the sertraline and placebo groups. One woman enrolled during two different pregnancies. She was assigned to placebo during the first (and suffered a recurrence of depression at week 6) and to sertraline during the second (she became hypomanic at week 11).
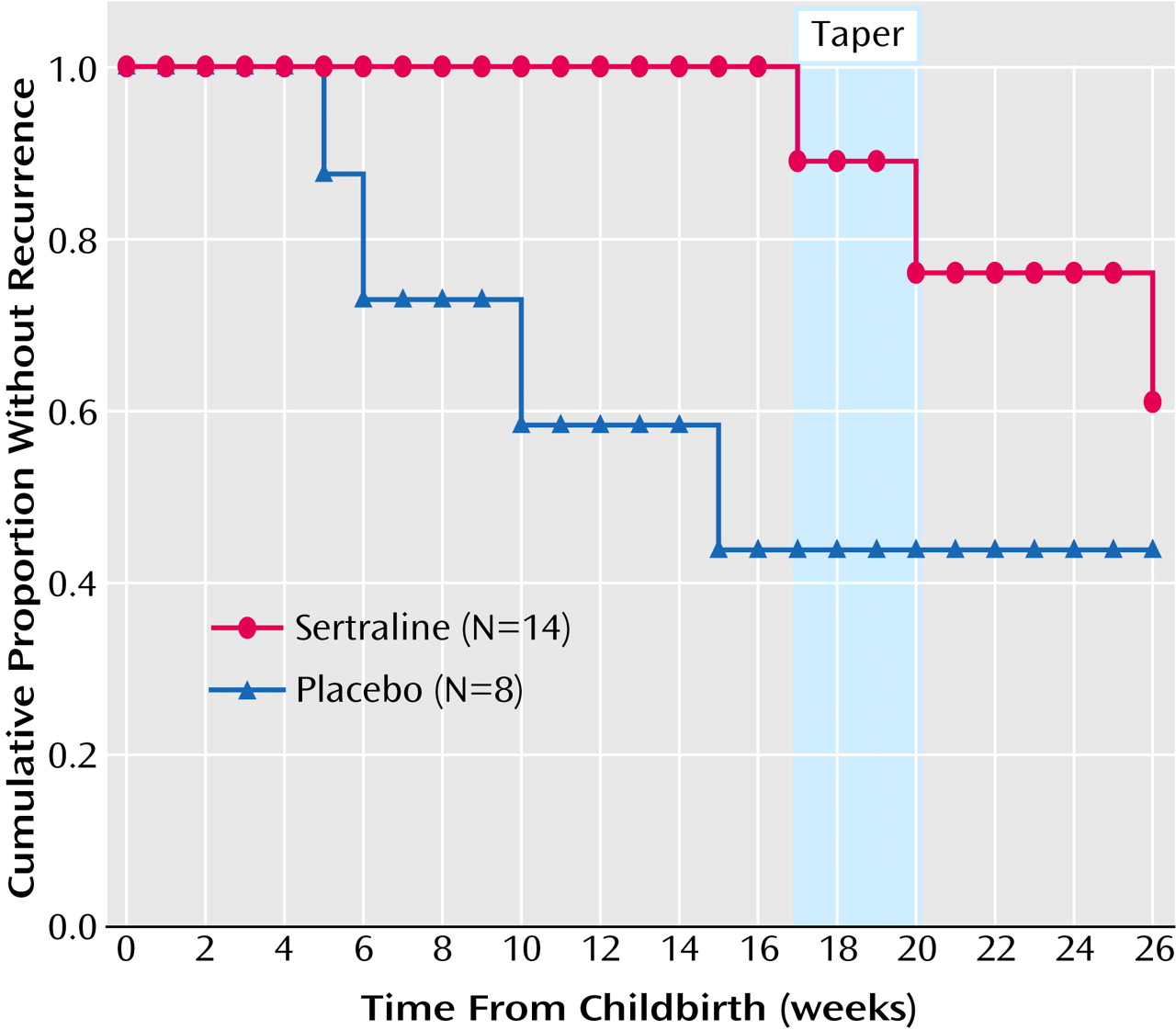
Hypothesis 1 was confirmed (
Figure 1). In our study group of 22 women, there were five recurrences in the 17-week preventive treatment period. These occurred in four of the eight women taking placebo (proportion, 0.50; 95% exact confidence interval [CI], 0.16–0.84) and in one of the 14 women taking sertraline (proportion=0.07, 95% exact CI=0.00–0.34) (p=0.04, Fisher’s exact test). The observed difference in recurrence rates was 0.43 (95% exact CI=–0.01 to 0.84). Because all of the women were compliant with medication, the intent-to-treat and reported analyses were equivalent.
Hypothesis 2 was also confirmed. The time to recurrence differed between the sertraline and placebo treatments (exact Wilcoxon-Gehan p=0.02). The observed hazard ratio was 0.11 (95% exact CI=0.02–1.02). Because one woman contributed information from two pregnancies, we analyzed the recurrence information with data from her second postpartum period removed. The analysis continued to yield a significant result (Wilcoxon-Gehan p=0.02).
In the 14 women assigned to sertraline, the one recurrence became manifest at week 17. Two women withdrew rapidly because of headaches, which resulted in the dose change already described. One subject was removed because of hypomania, and nine women completed the randomized clinical trial without recurrence. It is interesting that two of the nine women taking sertraline who completed the randomized clinical trial became depressed as the drug was tapered (week 20) or shortly after discontinuation (week 26).
The women assigned to sertraline reported dizziness and drowsiness more often than did the women who took placebo. The rates of dizziness were 57% (eight of 14) and 13% (one of eight) for sertraline and placebo, respectively (p=0.05, Freeman-Halton extension of Fisher’s exact test), and the rates of drowsiness were 100% (14 of 14) and 50% (four of eight) (p=0.02, Freeman-Halton extension of Fisher’s exact test).
Discussion
Sertraline prevented recurrence of postpartum-onset major depression better than did placebo, and the length of time to recurrence was significantly greater in sertraline-treated than in placebo-treated women. We selected 17 weeks as the duration of treatment to cover the risk period defined in epidemiologic studies
(2). When the sertraline was tapered, two women became depressed within a 6-week period. Sertraline prevented the
expression of postpartum-onset major depression, but the vulnerability to depression was manifest after the drug was withdrawn. Preventive antidepressant treatment should be provided for longer than 17 weeks. Our data suggest a minimum period of 26 weeks, consistent with treatment guidelines for a single episode of depression
(7).
These positive results contrast with those of a similar protocol that used the tricyclic nortriptyline
(1). The recurrence rates from these studies differed not only in the drug but also in the placebo conditions. In the earlier study, the recurrence rates were 23% for nortriptyline and 24% for placebo; in the current study, the rates were 7% for sertraline and 50% for placebo. We weighted the results of the present study by a factor of 2 to correct for the number of observations being half that in the nortriptyline trial. Combining the placebo groups from the nortriptyline and sertraline studies, we found a significant difference between the combined placebo group and the sertraline-treated women (hazard ratio=0.20, 95% CI=0.05–0.87, likelihood ratio chi-square, df=1, p=0.03) but not between the combined placebo group and the nortriptyline-treated women (hazard ratio=0.60, 95% CI=0.23–1.56, likelihood ratio chi-square, df=1, p=0.29). Placebo response rates are highly variable and increasing across time
(8). The placebo group contemporaneously associated with either sertraline or nortriptyline treatment is the more valid comparison
(8), as we present here.
If the efficacy of sertraline in preventing postpartum-onset major depression is confirmed in additional randomized clinical trials, the reason an SSRI prevented postpartum-onset major depression while a tricyclic did not must be considered. SSRIs increase brain levels of neuroactive steroids, which may decrease the risk for depression in the postpartum milieu
(9).