I keep the subject of my inquiry constantly before me, and wait till the first dawning opens gradually, by little and little, into a full and clear light.
Attempts to understand the genetic influences on schizophrenia go back to the roots of modern psychiatry. This effort has had four overlapping historical phases. The first confirmed the clinical impression of Kraepelin that schizophrenia “ran in families.” Uncontroversially, several generations of family studies of increasing methodologic sophistication verified that first-degree relatives of individuals with schizophrenia have an approximately 10-fold increased risk of illness. The second phase determined whether this familial aggregation was due to genetic or environmental factors. While more controversial, evidence from a series of adoption and twin studies showed that genetic factors contribute strongly to the etiology of schizophrenia.
These first phases assessed the aggregate effects of genes averaged over the genome. By contrast, the third or “gene localization” phase has used linkage to attempt to localize susceptibility genes to specific chromosomal regions. While findings from the first two phases of schizophrenia genetics were marked by substantial cross-study consistency, linkage studies of schizophrenia produced inconsistent results and poor rates of replication. Two findings suggest that, despite methodologic difficulties, linkage analysis can produce replicable results in schizophrenia. First, random a priori subsets of the Irish Study of High-Density Schizophrenia Families produced replicable evidence for linkage in three of four major genomic regions
(2). More important, a careful meta-analysis of 20 schizophrenia genome scans produced evidence that these studies agreed, much more than would be expected by chance, on those regions that likely contained susceptibility genes
(3).
I outline here recent exciting developments in the fourth phase of schizophrenia genetics, which is attempting to identify specific susceptibility genes. This story begins in 1995 when, during the first stages of analysis of the Irish Study of High-Density Schizophrenia Families, linkage was detected on chromosome 6p24-22
(4). Evidence for linkage in this region, which was replicated by some but not other groups
(5,
6), was in aggregate sufficient to initiate a fine mapping project in the Irish sample under one part of this broad linkage peak. Family-based analyses produced rather clear evidence for an association between schizophrenia and a set of DNA markers in the gene DTNBP1 (
dys
trobrevi
n binding
protein 1) or dysbindin-1
(7). Dysbindin-1, a 40–50-kDa protein that is expressed in neurons in many areas of the mouse and human brain, is named for its capacity to bind dystrobrevins
(8,
9), proteins that are part of the dystrophin glycoprotein complex
(10).
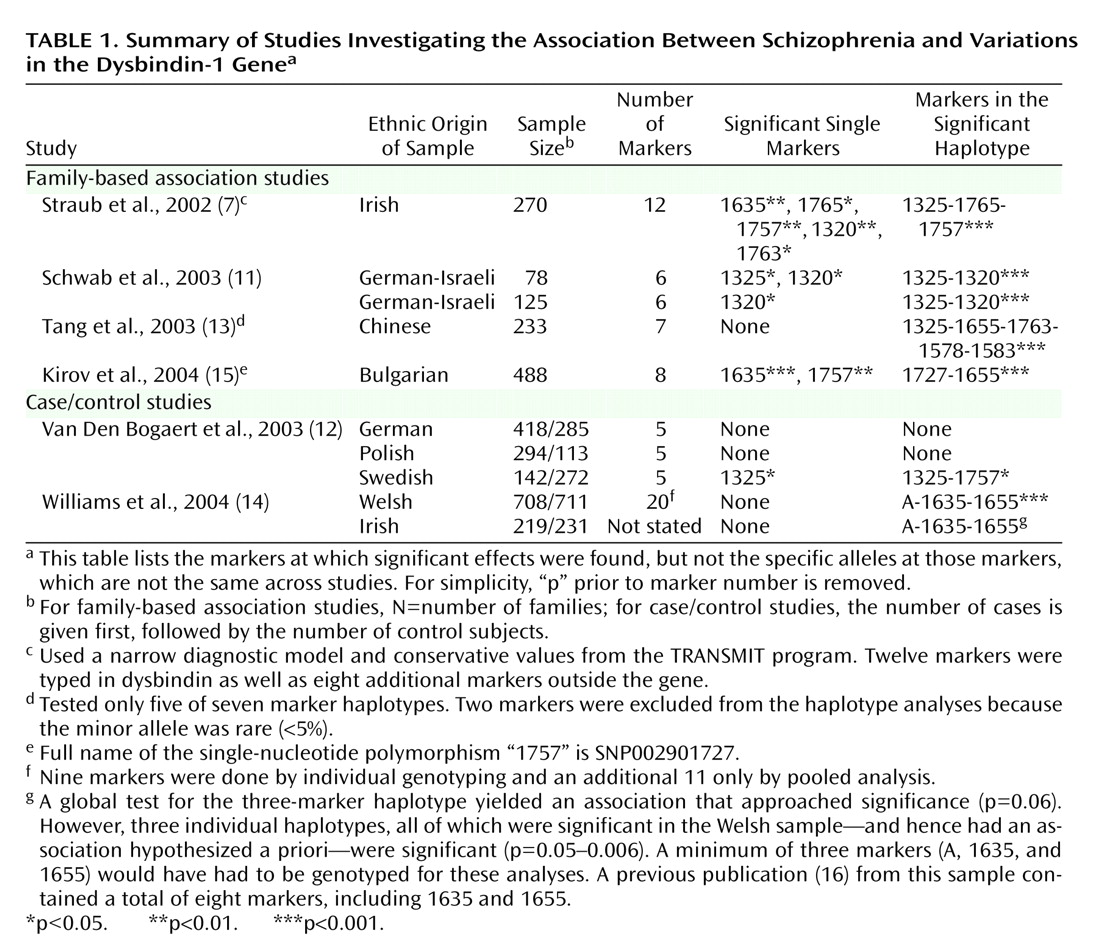
Since publication of this report in July 2002, replication efforts have been published from nine samples in five reports
(11–
15) (
Table 1). (In addition, one negative study
[16] was superseded by a positive report
[14] with typing of additional markers, and further analyses in the Irish Study of High-Density Schizophrenia Families defined more clearly a high-risk haplotype
[17].)
Table 1 reports results for individual markers and for haplotypes. Haplotypes are distinctive blocks of DNA, identified by a particular set of alleles at a group of markers, that are so small that they are nearly always inherited together as a unit. (A marker specifies a location on the DNA molecule, while the allele indicates the particular base pair at that location.) Generally, haplotypes provide more power for association studies than do single markers.
Using single markers and haplotypes, significant associations were found in, respectively, four of nine and seven of nine of the replication samples. Between-study variation was found both in the significant single markers and the most significant haplotype. In addition, the alleles at the markers that were associated with schizophrenia were not identical across samples.
We have little experience in the interpretation of such data. Each study had a clear a priori prediction: markers or haplotypes in the dysbindin gene would be associated with schizophrenia. However, multiple tests were made of individual markers and haplotypes. Publication bias cannot be ruled out—although other positive reports have been presented in abstract form
(18). The most important question we can ask is, given these findings, what is the probability that the hypothesis first put forward in the original report
(4)—that variation in the dysbindin gene influences risk for schizophrenia—is false? No definitive answer to this critical question is yet possible. Prudence is suggested given the poor prior track record of claims for single gene effects in psychiatric illness. However, in aggregate, the results are strongly suggestive and argue that it is unlikely, and perhaps highly unlikely, that the original report was in error.
A secondary but still important question: if dysbindin is a susceptibility gene for schizophrenia, what can we say about its effect size? Not counting the original report (where the effect size might be biased upward), odds ratios reported or calculated from replication reports were 1.87 (marker 1325 combined across both samples
[11]), 1.70 (marker 1325 in the one positive sample
[12]), 1.23 (high-risk haplotype
[13]), 1.40 (high-risk haplotype combined across both samples
[14]), and 1.58 (marker 1635
[15]). If dysbindin is a susceptibility gene for schizophrenia, it is likely to be of small to moderate effect and account for a modest proportion of total genetic risk.
Very recently, two postmortem studies have added intriguing new information to the dysbindin-schizophrenia story. Talbot et al.
(18) studied postmortem brains of two sets of matched pairs of schizophrenia and normal comparison individuals (N=17 and N=15, respectively). Using immunohistochemical methods, they found, in both samples, significant reductions of presynaptic dysbindin-1 in the hippocampal formation of the schizophrenia subjects, in particular in the terminal fields of intrinsic, glutamatergic afferents of the subiculum, the hippocampus proper, and the inner molecular layer of the dentate gyrus. These results were unlikely to result from neuroleptic exposure. Weickert et al.
(19) studied levels of dysbindin messenger RNA in postmortem brains for 15 normal subjects and 14 individuals with schizophrenia. Dysbindin mRNA was widespread in the brain and was significantly reduced in multiple layers of the dorsolateral prefrontal cortex in schizophrenia subjects. This reduction was specific and not seen with other markers of synaptic integrity. They also reported that dysbindin mRNA levels were significantly correlated with four of 11 markers in the dysbindin gene.
In aggregate, these results are exciting, but it is important to emphasize what we do not know about the schizophrenia-dysbindin connection. We have no clear molecular “smoking gun”—a DNA variant that is both associated with schizophrenia and produces a distinct change in dysbindin structure or function. We have only the dimmest idea of how variation in dysbindin might contribute to the pathophysiology of schizophrenia. Given that only a subset of individuals with schizophrenia carry the high-risk dysbindin haplotype, why were reduced levels of dysbindin seen in the hippocampus of nearly all affected cases
(18)? We do not have a single high-risk haplotype, and it remains unclear how to understand differences across samples. While multiple pathogenic mutations in dysbindin could explain these findings, it is perplexing to have such divergent results from ethnically similar populations.
So perhaps a corner has been turned in our long struggle to understand the genetic basis of schizophrenia. Furthermore, dysbindin is not the only gene where replicated evidence for association is emerging (see reference
6 for a recent review). For the genetics of schizophrenia, it may no longer be outlandish to hope that we will see “the first dawning [which] opens gradually, by little and little, into a full and clear light.”