From the time of Alzheimer’s first description of psychotic symptoms in a patient with Alzheimer’s disease in 1907, psychosis has been recognized as a major clinical syndrome in this illness. The consequences of psychotic symptoms in Alzheimer’s disease may be painful and costly for the affected individuals, those who care for them, and society at large. Psychotic symptoms have been linked to greater caregiver distress
(1–
3) and have been found to be a significant predictor of functional decline and institutionalization
(4–
7). Compared to patients with Alzheimer’s disease without psychosis, those with Alzheimer’s disease and psychotic symptoms are also more likely to have worse general health
(8) as well as a greater incidence of other psychiatric and behavioral disturbances
(9–
11). Psychotic patients tend to have more frequent and problematic behaviors, including agitation
(12–
14), episodes of verbal and physical aggression
(10,
15–18), and anxiety
(11).
Reviews completed before the early 1990s found that psychotic symptoms were common in dementia, including Alzheimer’s disease
(19–
23). In their review of 21 studies, for example, Wragg and Jeste
(23) found that approximately one-third of all patients with Alzheimer’s disease had delusions at some point during their illness, 28% had hallucinations, and nearly 35% had other psychotic symptoms that were difficult to categorize. Overall, however, the reviewed studies were compromised by sampling deficiencies and methodological problems. Wragg and Jeste’s review included studies with as few as nine subjects. Moreover, only five of the 21 studies had a sample size larger than 100 subjects. Other methodological problems included the use of unreliable or nonvalidated diagnostic criteria for Alzheimer’s disease. Consequently, samples included individuals with various types of dementias, and thus generalizability was limited, and findings as they related to Alzheimer’s disease specifically were obscured. Imprecise operational definitions of psychosis
(24) and utilization of assessment methods with questionable reliability and validity also undermined these investigations. Moreover, all of the studies published before 1990 were cross-sectional or descriptive and thus did not provide data on the incidence or course (e.g., persistence) of symptoms.
Since the early 1990s, research on psychosis of Alzheimer’s disease has advanced considerably. There have been improvements in the development of diagnostic criteria for Alzheimer’s disease and for psychosis of Alzheimer’s disease
(25) and the development of more reliable measures of psychotic symptoms, including the Behaviorial Pathology in Alzheimer’s Disease Rating Scale
(26) and the Neuropsychiatric Inventory
(27). Larger sample sizes have become available because of increased awareness of the disease and the establishment of Alzheimer’s disease centers. Longitudinal data from these centers have become available, and more investigators have undertaken prospective studies on this topic.
We reviewed studies published from 1990 through 2003 that investigated psychosis of Alzheimer’s disease with the aim of providing a systematic overview of the current state of knowledge in this area. In so doing, we employed more stringent inclusion criteria than were applied in reviews conducted before the early 1990s. In this article, we summarize findings on the epidemiology of psychotic symptoms in Alzheimer’s disease. Delusions and hallucinations are also reviewed separately, and we include findings on other uncategorized psychotic symptoms. In addition, we examine the literature on potential risk factors for psychosis of Alzheimer’s disease. Implications of the findings for clinical practice and for future research are discussed.
Discussion
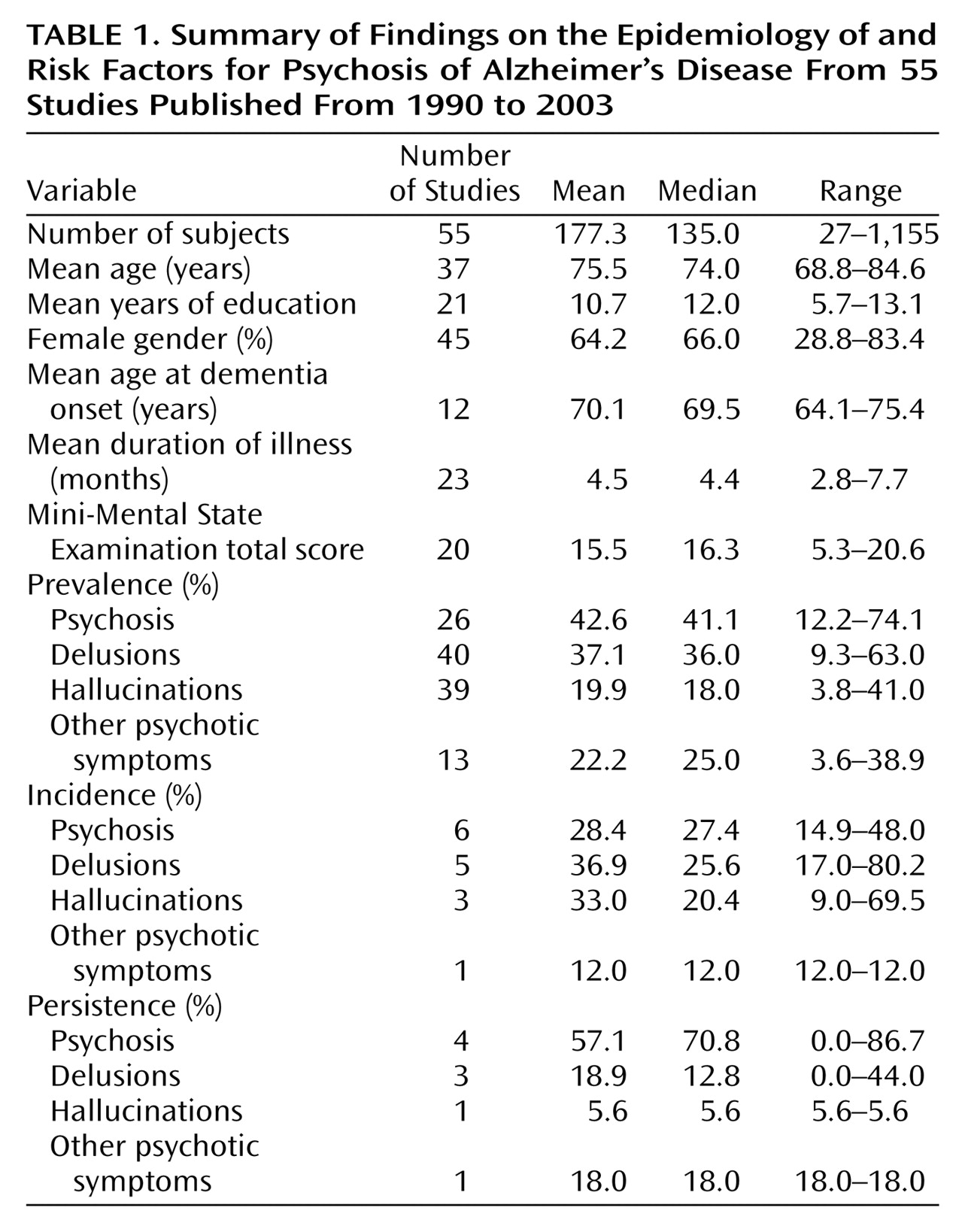
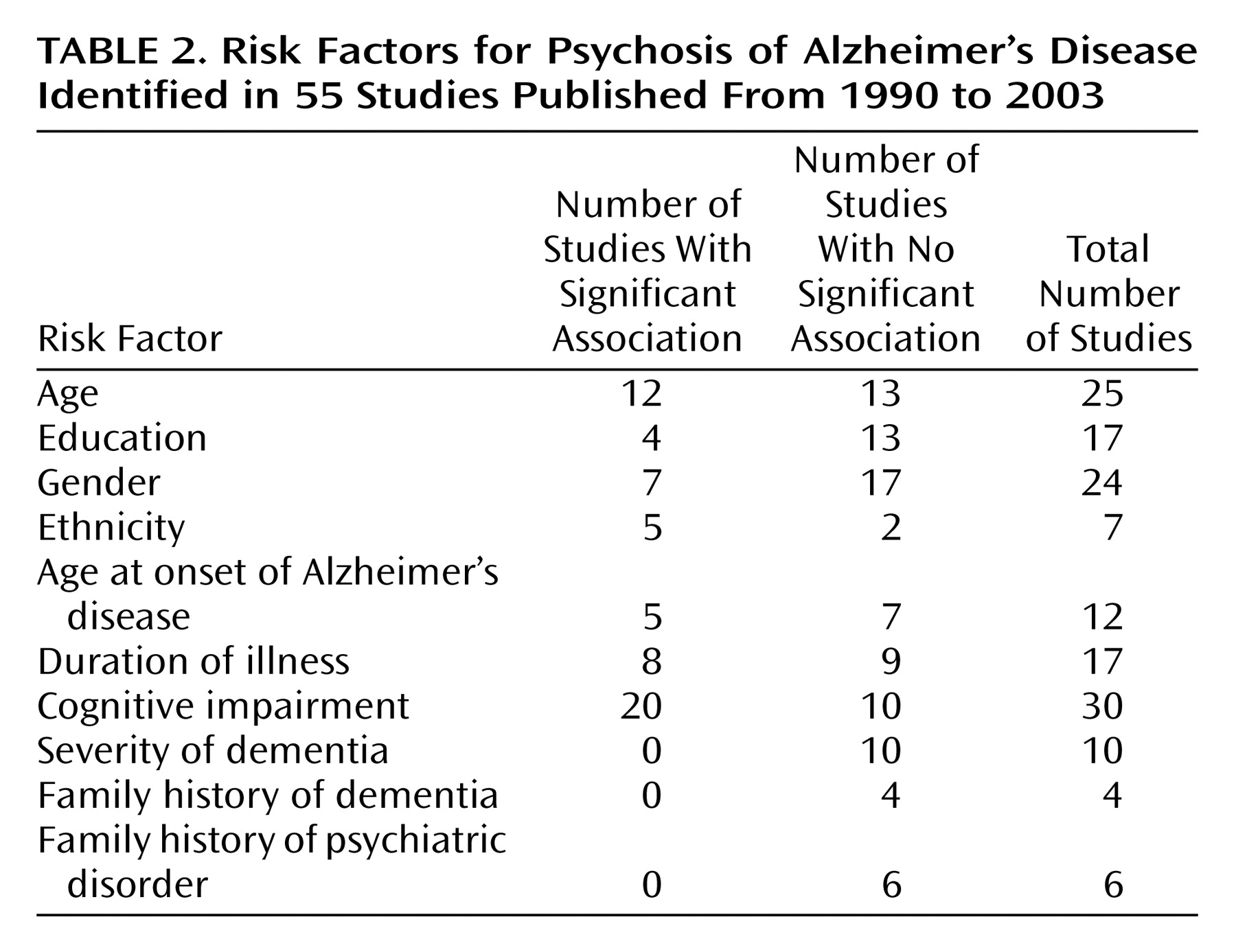
Our review of 55 studies of psychosis in possible or probable Alzheimer’s disease revealed that a sizable proportion (median 41%) of individuals with the disease experience psychotic symptoms at some time during the course of their illness. Delusions occurred more frequently (median=36%) than hallucinations (median=18%). Other psychotic symptoms not categorized as delusions or hallucinations occurred in 25% of individuals. The incidence of psychotic symptoms seemed to increase with increasing follow-up intervals over the first 3 years. Psychotic symptoms tended to be reported in a majority of patients at least over a period of several months but often were not observed beyond 1 or 2 years. African American or black ethnicity and greater degree of cognitive impairment were strongly associated with a higher rate of psychosis. Psychosis was also associated with a faster rate of cognitive decline. Age, age at onset of Alzheimer’s disease, and duration of Alzheimer’s disease were associated with psychosis in approximately one-half of studies. Education, gender, and family history of dementia or psychiatric illness showed a weak or inconsistent relationship with psychosis in patients with Alzheimer’s disease.
The prevalence rate of psychosis in patients with Alzheimer’s disease found in our review was 41%. The median rate for delusions was 36%, which is comparable to the median rate of 33.5% reported in one of the only review studies of psychosis in Alzheimer’s disease published before the early 1990s
(23). The rate of hallucinations found in the present review (18%) represents a decrease from the 28% reported by Wragg and Jeste
(23). The fact that prevalence remains high in light of pharmacologic treatment may reflect increased awareness that these disturbances are consequences of Alzheimer’s disease, improved detection, or the use of better criteria and rating scales that allow for psychotic symptoms to be diagnosed with greater accuracy. As an increasing number of patients with Alzheimer’s disease are treated with cholinesterase inhibitors over the coming years, we might expect that the prevalence and incidence of psychosis would decrease, although findings for the efficacy of these drugs in reducing psychotic symptoms specifically have been mixed (see references
13,
82,
83).
The fact that psychosis is persistent over a short interval of a few months may reflect the reasonable amount of time it takes to begin typical treatment for psychosis and to observe amelioration of symptoms. To assess the true persistence of symptoms, subjects would have to be enrolled in a placebo-controlled study in which some psychotic patients did not receive the typical treatment for symptoms. In the studies that were reviewed, it was more the exception than the rule that subjects would be excluded if they were taking an antipsychotic drug or cholinesterase inhibitor or that a drug washout period would be invoked. Furthermore, there were no means of determining whether the patients who were taking these drugs were being treated optimally, and the extent to which psychotic symptoms persist despite antipsychotic treatment is not known. Therefore, persistence values may reflect the experience of psychosis given current treatments rather than the true persistent nature of psychotic symptoms.
Few equivocal associations with psychosis emerged from the reviewed studies. The association between African American or black ethnicity and psychosis is intriguing, although it is also limited by the fact that only Caucasian samples are available for comparison. Issues of acculturation and genetic influences are yet to be adequately examined, highlighting an area in need of exploration. Cognitive impairment and the rate of cognitive decline were also found to be strongly associated with psychotic symptoms.
The findings of the present review suggest that psychosis represents a developmental feature marking the progression of Alzheimer’s disease or that it represents a distinct disease subtype marked by psychotic symptoms and a particularly rapid disease course. The fact that delusions, specifically, seemed most prevalent in patients with moderate cognitive impairment supports the hypothesis that a certain amount of neuronal integrity must be present for delusions to occur (see references
48,
84). Conclusions are limited, however, by a general failure to include severely cognitively impaired subjects in these studies. In addition, the association between psychosis and cognitive impairment and between psychosis and rate of cognitive decline may be influenced by medications, including antipsychotics and cholinesterase inhibitors, the former of which is recommended as a first-line treatment for dementia patients with delusions
(85). Yet, a majority of the studies reviewed did not account for the potential effects of medication on cognition and simply reported that these effects were a possible limitation to their findings. A number of studies altogether failed to report what, if any, medications the subjects were taking. The importance of considering medication effects is illustrated in studies of antipsychotic use and cognition. The use of two atypical antipsychotics (clozapine and risperidone) in cognitively impaired patients was reviewed by Jeste et al.
(86) and Gladsjo et al.
(87). Jeste and colleagues found that the effects of clozapine on cognition were somewhat conflicting, which they posited was due, at least in part, to the strong anticholinergic activity of clozapine, which is likely to confound or diminish any enhancement of cognitive functioning. Berman and colleagues
(88,
89) reported significant increases in MMSE scores in patients with schizophrenia or mild dementia treated with risperidone. Moreover, cholinesterase inhibitors have been shown to improve cognitive symptoms or temporarily reduce the rate of cognitive decline
(90). Certainly, future studies should examine the potential influence of medication use, not only to examine any potential effects, positive or negative, on cognitive functioning but also to elucidate underlying biological mechanisms of psychosis in dementia. Furthermore, difficulties in diagnosing patients with Lewy body dementia may have led to their inadvertent inclusion in studies of patients with Alzheimer’s disease, thereby affecting the association between some psychotic symptoms and rate of cognitive decline, given that psychotic symptoms, and hallucinations in particular, may occur in nearly one-half of those with Lewy body dementia
(30,
91).
For many variables that were found not to be associated with psychosis, including age, age at onset, and duration of illness, small standard deviations likely affected the detection of associations. In the case of age and age at onset, few individuals who were younger than age 55 years or who had an early age at onset (age 55 years or younger) were included in these studies. Similarly, the range and standard deviation for illness duration were restricted (range=2.8–7.7 years, SD=1.33 years), thus limiting the potential to detect a positive association. In addition, many authors noted that age at onset of Alzheimer’s disease was inherently difficult to determine, because it was often an estimate that relied on the failing memory of those with Alzheimer’s disease or the recall and dating by others of behaviors that occurred several years earlier.
The results of this review are also limited by problems in assessing psychosis. Despite more regular use of accepted diagnostic criteria, some researchers continue to use diagnostic criteria that are nonspecific to Alzheimer’s disease (e.g., DSM-III or DSM-IV criteria). Even when accepted criteria are utilized, inconsistencies in interpreting those criteria are evident. Presumably, the rates reported herein may underestimate the prevalence of delusions and hallucinations specifically, as evidenced by the fact that from 3.6% to 38.9% of psychotic symptoms remained uncategorized and were labeled “other psychotic symptoms.” Conversely, as suggested by Devanand and colleagues
(24), the lack of clarity may result in an overestimation of prevalence rates for symptoms such as delusions, as some symptoms are classified as delusions when they would otherwise be better classified as other psychiatric symptoms or as behavioral problems of Alzheimer’s disease. Clarity regarding the definition of psychosis and the categorization of symptoms such as misidentifications will be necessary to produce data that can be better compared across studies.
Overall, the present review reflects improvements in sampling, study design, diagnosis, and assessment, compared to reviews conducted before the early 1990s. Subject samples were larger, providing a more accurate picture of the nature and frequency of psychosis. More studies were prospective in nature and thus used methods designed to answer a directed research question. Longitudinal data were more readily available, providing information on incidence that had not previously been reported and other insights into how psychotic symptoms affect the course of Alzheimer’s disease over time. More reliable assessment tools have also come into use over the past decade with the advent of measurements such as the Neuropsychiatric Inventory and Behaviorial Pathology in Alzheimer’s Disease Rating Scale and the use of structured clinical interviews, as opposed to the previously employed methods of chart review and behavioral observation. However, future studies should continue to address the remaining shortcomings of the past 15 years. Research should use longitudinal designs to advance our understanding of the incidence and persistence of psychosis. Future studies should also develop or utilize appropriate diagnostic criteria and rating scales for psychosis in the Alzheimer’s disease population. By taking into account medication use (such as antipsychotics and anticholinergics) among subjects included in these studies, we may also learn about the relative benefits of various pharmacological agents in treating psychosis as well as the mechanisms underlying the occurrence of psychotic symptoms in Alzheimer’s disease and other illnesses.