Depression is potential risk factor for increased morbidity and mortality across numerous medical conditions
(1), including HIV and AIDS
(2–
4). In chronic HIV disease, depression may adversely affect quality of life and adherence to medication regimens
(5,
6), which may subsequently affect disease progression and health outcomes. Our work has suggested that killer lymphocytes may be altered during depression and subsequently affect the HIV viral load
(7), although the exact mechanisms involved are not yet delineated.
Depressive symptoms are frequently reported among HIV-seropositive individuals, and several studies have documented elevated rates of major depression and subclinical depressive symptoms among HIV-seropositive homosexual men
(8–
12), although these rates are similar to those of HIV-seronegative homosexual men in the general population. The majority of these early studies of the prevalence of depression focused almost exclusively on men because of the demographics of HIV disease at the time. However, women now account for just over 50% of those infected with HIV globally, and the rates of new HIV cases among women in the United States and many other nations are rising
(13). Because medically healthy women in the general population are also diagnosed with depression more often than men
(14), it is increasingly salient to study the prevalence and effects of depression among HIV-seropositive women as well.
Recent studies have reported increased rates of the prevalence of depression and other mood disturbances among HIV-seropositive women. Morrison and colleagues
(15) examined the prevalence of depressive disorders among 93 HIV-seropositive and 62 HIV-seronegative women and found that HIV-seropositive women without an active substance abuse problem had a significantly higher prevalence rate of major depressive disorder (19.4%) than HIV-seronegative women (4.8%). In a prospective longitudinal cohort study of 765 HIV-seropositive women, 42% reported chronic depressive symptoms, and 35% had intermittent depressive symptoms
(2). In this study, depression was also associated with markers of the progression of HIV disease and mortality. Although the exact mechanisms underlying the association between depression and disease morbidity and mortality are unknown, the effects of depression on the immune system may represent a key mechanistic pathway
(16,
17).
Associations between depression and immune system impairments among medically healthy individuals have been observed. The severity of depression has been associated with decrements in several in vitro measures of immunity (e.g., lower CD8+ T [antigenic marker on suppressor/cytotoxic T] cells and natural killer [NK] cell numbers and activity) in a large-scale meta-analysis
(18). Patients diagnosed with depression, especially severe depressive states, also have pronounced decrements in immunity
(19). Evans et al.
(20) examined the effects of major depression on peripheral blood NK cell phenotypes and NK cell activity among depressed and nondepressed comparison participants. Depressed subjects exhibited significant reductions in NK effector cell numbers, Leu-11 (CD16) and Leu-7 (HNK-1), and NK cell activity.
Studies examining the association between depression and immunity, particularly CD4+ T lymphocyte counts, in HIV disease have reported both positive and negative findings
(21–
26). These results may be due to differences in psychiatric assessments or the patient samples studied or perhaps to a reliance on broad indices of immune status (e.g., CD4+ T cell counts), which may not be the most sensitive or reliable indicators of immunity. Depression is associated with alterations in more specific measures of immune function among HIV-seropositive women. For example, depression was associated with decrements in killer lymphocyte function among a group of 63 HIV-seropositive women
(7). These measures are a specific indicator of innate immunity, which may be relevant to the progression of HIV disease; however, to our knowledge, no study to date has examined the association between changes in depression status and NK cell activity over time among HIV-seropositive women.
Irwin and colleagues
(27) assessed NK cell lytic activity at intake and at a 6-month follow-up among medically healthy depressed individuals and comparison subjects in a longitudinal case-control design and found that as depression scores decreased, NK cell activity increased in the depressed subjects, but neither changed in the comparison subjects. Frank et al.
(28) examined medically healthy depressed individuals and found that 4 weeks of fluoxetine treatment was associated with augmented NK cell activity in a subgroup of subjects that initially exhibited low NK cell activity. Schleifer and colleagues
(29), however, found no change in NK cell numbers or cell activity among 21 medically healthy adults with major depression after 6 weeks of antidepressant treatment, although they did find both decreased NK cell numbers and function during initial depressive states. One way to reconcile these differences is to investigate subjects over longer time intervals with more specific immune assays, which may allow for more opportunities to observe alterations in depression and more precise changes in immunity.
In the current study, we assessed the status of a diagnosis of major depression and immune system functioning in a group of HIV-seropositive women over a period of up to 2 years. We predicted that resolution of the major depression and improvement in acute depressive symptoms would be significantly associated with increases in NK cell activity over time. We further hypothesized that a subgroup of women who showed significant improvements in mood (as indicated by a resolution of a major depression diagnosis) over time would also show improvements in immune functioning, as measured by the specific functional measure of innate immunity, NK cell activity. We also predicted that a subgroup of HIV-seropositive women who did not meet the diagnostic criteria for major depression would show no significant changes in NK cell activity.
Method
The data for this study were collected from two sites (Gainesville, Fla., and Philadelphia) as part of a prospective, longitudinal cohort study investigating the neuropsychiatric, endocrine, and immune aspects of HIV infection in women
(7). The current study specifically examines the association between depression and immune data obtained across a 2-year period.
Participants
HIV-seropositive women were recruited from outpatient clinics, public health departments, and other organizations focused on HIV disease and care through a combination of community presentations, clinician referrals, word of mouth, and media advertisements. The specific inclusion and exclusion criteria were detailed in a previous report
(7). Fifty-seven HIV-seropositive women had complete depression and immune data measured from at least two time points during the study period and thus were available for inclusion in the current main analyses. These 57 women were comparable to women without such matched data on relevant demographic, medical, and depression data
(7). To further understand whether NK cell activity, measured in lytic units (LU), changes with resolution in the clinical diagnosis of major depression, we examined participants who experienced a change in diagnosis over the follow-up period. Eleven of the 57 women were diagnosed with major depression either at study entry or early on (i.e., within the first year of the study), but they did not meet criteria for major depression at the follow-up assessment. Forty-three women did not meet the criteria for major depression at the initial and final assessment time points, and three women who were not initially depressed became depressed. We focused this part of the investigation on the women whose depression resolved over time (N=11).
HIV serostatus was confirmed by using an enzyme-linked immunosorbent assay with Western blot analysis for confirmation of the presence of anti-HIV-1 antibodies. All women were fully aware of their HIV-seropositive status at study entry.
Procedures
The institutional review boards of the University of Pennsylvania and the University of Florida approved the protocol. The participants were assessed at baseline and followed up every 6 months across the 2-year period. All participants provided written informed consent and were reimbursed for their time, travel expenses, and child-care expenses.
Psychiatric and Medical Assessments
Each participant received a thorough outpatient assessment at study entry and at follow-up over a 2-year period, which included a physical examination and a structured psychiatric interview. Current and lifetime DSM axis I diagnoses, including major depressive disorder, were assessed by a psychiatric clinician with a modified version of the Structured Clinical Interview for DSM-IIII-R (SCID)
(30). Consensus diagnoses were determined at team meetings; the clinicians’ rating/diagnosing was blind to patient immune status, and all immune assessments were performed with blinding for rating/diagnostic status. We also assessed acute symptoms of depression with the 17-item Hamilton Depression Rating Scale
(31). HIV medication status was coded as a categorical variable pertaining to whether or not the woman was taking antiretroviral medication or protease inhibitors.
Immune Assessments
To control for potential circadian effects on immunity, all participants were evaluated at the same time of day. Specifically, the participants were placed in a recumbent position, an intravenous line was started at approximately 9:00 a.m., and intravenous line patency was maintained with a slow normal saline drip. Blood was obtained approximately 1 hour later. Blood cell counts and flow cytometry panels were performed on peripheral blood samples, as detailed previously
(7). NK cell activity was assessed by using standard techniques established in our laboratory
(32). Lytic units/10
7 of peripheral blood mononuclear cells and lytic units/10
7 of NK cells were calculated with the method of Bryant et al.
(33) and Friberg et al.
(34). By measuring the percentage of CD16+/CD56+ cells in the preparation of peripheral blood mononuclear cells, we determined the lytic units of NK activity (LUNK) per NK cell
(35). Expressing the NK data as LUNK cell adjusts for differences in the percentage of NK cells (CD16+/CD56+) in the effector cell population. Thus, our primary outcome measure of innate immunity was expressed as LUNK cell activity. We took the log of LUNK to make the distribution more symmetric and to equalize variances for group comparisons.
Viral Assessments
Serum HIV RNA viral load was determined from archived samples with the Amplicor Monitor assay (Roche Diagnostics, Branchburg, N.J.). The lower limit of quantification for this assay is 400 copies per milliliter of blood. Because approximately half the group measured at this lower limit and standard transformations, such as the log, would not normalize the distribution, we analyzed viral load as a dichotomous variable. Thus, viral load was quantified as either nondetectable (less than or equal to 400 copies/ml) or detectable (greater than 400 copies/ml) in the current study.
Statistical Analyses
Two different analyses were performed to evaluate the overall association between different depression measures as the time-varying covariate and log (LUNK) cell activity over time as the longitudinal dependent variable. First, to analyze such associations for the entire group (N=57), we employed separate linear regression models for each depression measure with subject effects included as fixed effects to account for significant heterogeneity among the subjects with respect to both LUNK and the depression measure, which would cause confounding under a random effects model. The estimate of the longitudinal correlation was obtained from the estimated regression slope divided by the model-based standard deviations for log (LUNK) cell activity and the depression measure. Second, for the analysis of the 11 women who exhibited a change in depression status, we used Wilcoxon’s signed rank tests to examine whether there were changes in LUNK cell activity during the period depression resolved. Similar tests were performed for the remaining women across an equivalent period of time. Finally, we used Spearman’s r for continuous and ordinal outcomes and chi-square tests for binary outcomes to compare different groups of subjects with respect to demographic and medical variables. In all analyses, we adjusted for baseline viral load, antiretroviral medication use, antidepressant treatment status, and time interval (in months) between assessments.
Results
The demographic and behavioral characteristics of the 57 HIV-seropositive women with depression and immune data that were available from at least two time points were obtained from each site (Gainesville, Fla., and Philadelphia) and were comparable, as reported in our earlier work
(7,
15). The mean age of the women was 39.7 years (SD=7.1). About 58% of the women were African American, and 35% were Caucasian. The majority of the women (81%) were taking an established regimen of HIV medications, and half of the group had a viral load in the detectable range. CD3+/CD4+ T cell counts averaged 521.6 (SD=7.1) for the entire group. Thirty-seven (65%) of the 57 women had a prior history of major depression, as assessed by the SCID at the initial assessment. Nineteen percent of the women reported that they were given a prescription for an antidepressant medication at the initial assessment. There were no significant differences between the groups on the relevant demographic and medical variables examined in this study.
The average time interval (in months) between the initial assessment and the final assessment for the group of 57 woman was 12.74 months (SD=5.79), with an average time interval of 13.64 months (SD=6.62) for the group of 11 women who were depressed and later had a resolution and 12.28 months (SD=5.70) for the group of 43 nondepressed women. There was no significant difference in time interval between the groups (χ2=0.36, df=1, p=0.55).
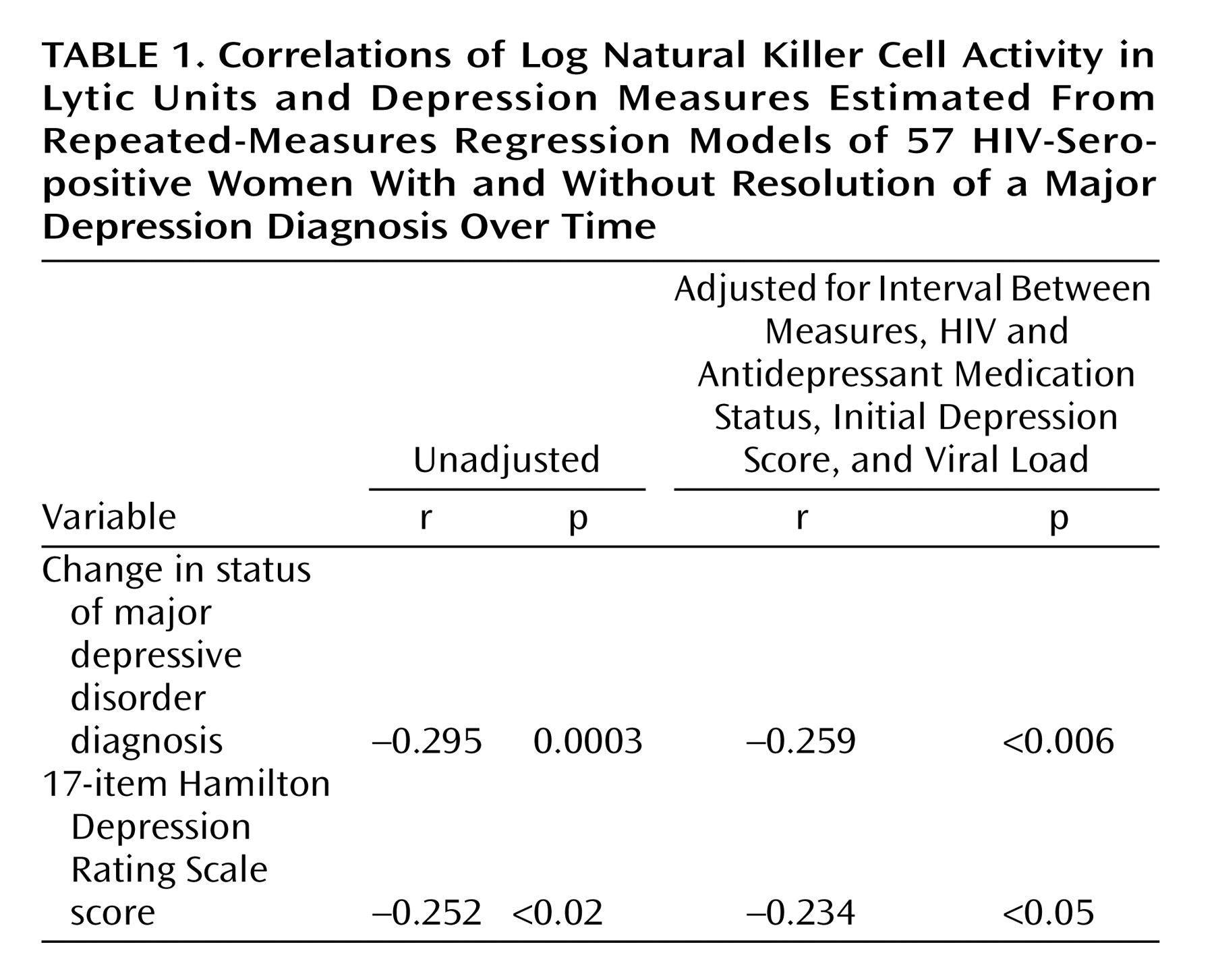
By employing linear regression with the subject as a fixed effect to evaluate associations between depression and immunity in the entire group (
Table 1), improvements in the diagnostic status of depression (r=–0.259, p<0.006) and decreases in Hamilton depression scale scores (r=–0.234, p<0.05) were significantly associated with increases in log (LUNK) cell activity over time. These associations were significant, even when we controlled for initial depression diagnostic status or Hamilton depression scale scores, respectively, as well as viral load, HIV medication status, antidepressant medication status, and the time interval between assessments.
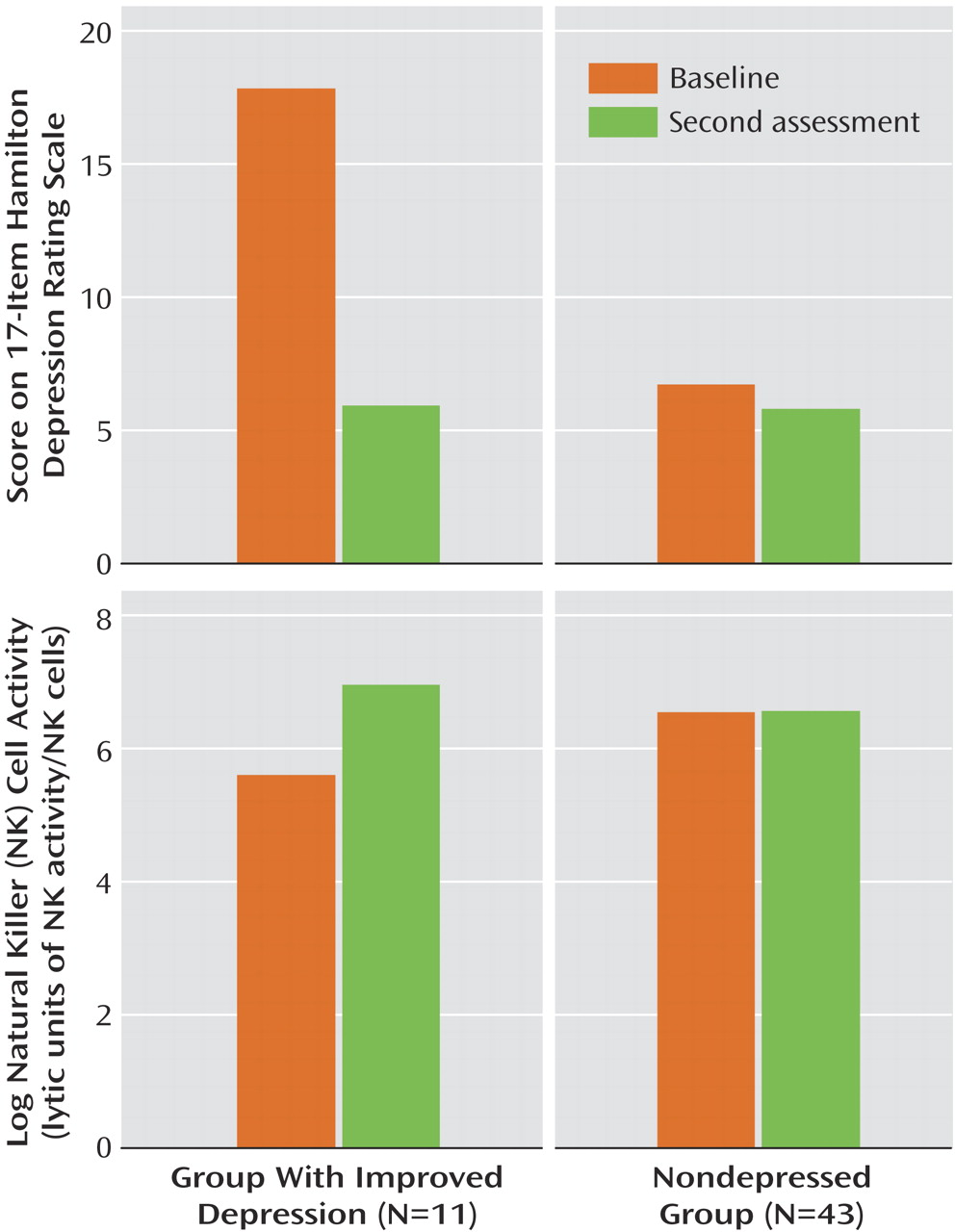
Hamilton depression scale scores paralleled major depression diagnostic status over time, improving significantly (mean=17.8, SD=6.5, to mean=5.9, SD=5.7) in the 11 women with a resolution of their major depression diagnosis (z=–32, p=0.002) and showing no significant change (mean=6.7, SD=5.9, to mean=5.7, SD=5.7, in the 43 women who were nondepressed (z=–53, p=0.42). In the group with improved depression, eight (73%) of the 11 women had remitted depression, as defined by a Hamilton depression scale score of 7 or less; one woman met criteria for dysthymic disorder but not major depression at the final assessment.
LUNK cell activity of the group with resolved depression (N=11) and the nondepressed group (N=43) of HIV-seropositive women over time is presented in
Figure 1. In the resolution group (11 of 57, 19.3%), representing women whose depression diagnostic status improved, there was also a significant increase (mean=5.6, SD=1.4, to mean=7.0, SD=1.0) in log (LUNK) cell activity over time (z=28, p=0.009). In fact, 82% (nine of 11) of the women in this group had improvement in log (LUNK) cell activity that paralleled resolution of their major depression diagnostic status. If we include the three women who entered the study as nondepressed but became depressed by their final assessment, along with the 11 women with a resolution of their depression diagnostic status, there remains a significant absolute change in log (LUNK) cell activity among these 14 women (z=43.5, p=0.004) over time. Of these 14 women who experienced a change in their major depression diagnostic status in either direction, 11 (79%) had a significant parallel change in log (LUNK) cell activity over time. In the nondepressed group (43 of 57, 75.4%), however, there were no significant changes (mean=6.6, SD=1.6, to mean=6.6, SD=1.4) in log (LUNK) cell activity (z=18, p=0.83) during an equivalent period.
Discussion
This study examined the association between the diagnostic status of major depression, acute depressive symptoms, and a specific measure of innate immunity—LUNK cell activity—over time among a contemporary group of HIV-seropositive women. The women whose depression diagnostic status resolved over time also showed a significant increase in LUNK cell activity across this same period. However, the women who did not meet the diagnostic criteria for major depressive disorder did not show a significant change in immunity during an equivalent period. This study is one of the first to demonstrate that an improvement in depression diagnostic status results in a parallel change in a specific indicator of immune system functioning relevant to progression of HIV disease. These findings extend our previous work demonstrating significant inverse relationships between depression and NK cell activity in a cross-sectional study
(7).
To our knowledge, this is also the first study in HIV disease to show that change in the diagnostic status of major depression and reduction in acute depressive symptoms are significantly associated with increases in LUNK cell activity over time. This association was observed even though the Hamilton depression scale scores of the women in our depressed group were in the moderate range, as observed in our earlier studies of HIV-seropositive women
(7,
15), which indicates that this association may be even stronger in more severely depressed groups. Our more direct measure of NK cell functional activity (LUNK) may be an underlying mechanism of the effects of depression on HIV morbidity and mortality observed in previous studies
(2–
4). Prior HIV studies relating depression and immunity have reported mixed results when the authors used more global indices of immune status, such as CD4+ T cell counts
(21–
26). The results of the current study point to the benefits of employing more specific indicators of immunity, such as LUNK cell activity, which are potentially relevant to the progression of HIV disease.
Clinical studies of depression in subjects without other medical illness have demonstrated significant alterations in NK cells
(20), a cellular immune population that may play a key role in regulating HIV infection. There is mounting evidence that NK cells exert anti-HIV effects by both classic killing activity as well as the production of HIV-suppressive factors. Specifically, NK cells may be involved in a natural resistance against viral infection and may have the capacity to lyse HIV-1 infected cells
(36–
40). In our studies of HIV-infected men, alterations of NK lymphocytes associated with stress and depression were observed
(23,
24), suggesting that killer lymphocytes mediate the effects of depression in the earlier stages of the progression of HIV disease. Ironson et al.
(41) observed that NK cell number and function are preserved among AIDS patients with low CD4+ T cell counts and stated that these immune factors may be important in maintaining the health and well-being of these individuals. Thus, the mechanism underlying the impact of depression on the progression of HIV disease may operate in part by altering NK cell numbers and activity.
There are a number of limitations to the current study. The design was a prospective cohort study and not a population-based study, and thus, a sampling bias may have resulted. Although recruitment was open to women of all races and ethnic backgrounds, African American and Caucasian participants comprised the majority of the participants. Thus, these findings may not be generalizable to Hispanic or Asian populations. We also excluded women who were currently abusing alcohol or other substances to reduce the confounding effects of these substances on depression and immunity. This may also limit the generalizability of our findings. This was an observational study, not a treatment study, so we did not have complete data on adherence to specific antidepressant treatment (either pharmacological or psychological), and thus, it is difficult to ascertain whether the alterations in depression observed were due to a specific treatment or simply to the passage of time. Regardless of how depression improved in these women, the associated enhancement in immunity is noteworthy and warrants more extensive clinical evaluation in future studies.
In conclusion, our findings provide the first evidence that resolution of major depression is associated with significant increases in NK cell activity over time in HIV-seropositive women. These results extend previous findings demonstrating depression-associated decrements in NK cell numbers and function and suggest that these alterations are reversible with the resolution of the depressive episode. Increasing evidence suggests that depression may have a negative impact on the progression of HIV disease, and chronic depression has been associated with mortality in HIV-seropositive women. Given the role that innate immunity plays in the host’s defense against HIV infection, future studies assessing antidepressant treatment effects could shed light on the relationship and underlying mechanisms of depression, immunity, and the progression of HIV disease.