There is a need to identify novel therapeutic targets for the anxiety disorders
(1). Recent studies in generalized anxiety disorder have investigated nonbenzodiazepine anxiolytic medications that primarily modulate the γ-aminobutyric acid (GABA) system
(2,
3), but the role of the excitatory amino acid glutamate has been less scrutinized. Preclinical and clinical studies suggest abnormalities in glutamate regulation in anxiety disorders and the anxiolytic potential for antiglutamatergic agents. Nonhuman primate studies of early-life stress have shown increased glutamate activity in brain regions implicated in anxiety or fear responses
(4). It has also been shown that antiglutamatergic agents suppress stress hormonal responses in nonhuman primates
(5) and facilitate the extinction of conditioned fear in rodents
(6).
Riluzole, a presynaptic glutamate release inhibitor approved by the U.S. Food and Drug Administration for the treatment of amyotrophic lateral sclerosis, was recently shown to have antidepressant and anxiolytic properties in treatment-resistant major depression
(7,
8). To test the idea that an antiglutamatergic medication would be effective for primary anxiety disorders, we conducted an open-label, proof-of-concept investigation of riluzole in patients with generalized anxiety disorder.
Method
Eighteen adult outpatients (six men, 12 women; mean age=33.6 years, SD=9.4) were recruited through media advertisement or flyers. All patients met DSM-IV criteria for a current diagnosis of generalized anxiety disorder, confirmed by an independent evaluator using the Structured Clinical Interview for DSM-IV. Mean duration of illness was 14.5 years (SD=9.6). Comorbid conditions included panic disorder (N=7), dysthymia (N=6), social anxiety disorder (N=4), and specific phobia (N=2). Exclusion criteria consisted of major depressive episode or substance abuse/dependence (other than nicotine) within 6 months of study entry; lifetime history of psychotic, bipolar, obsessive-compulsive, posttraumatic stress, or eating disorder; mental retardation or learning disability; autism; or substantial medical or neurological conditions requiring medication.
Five patients had never received psychotropic medication, and all patients had been free of psychotropic medication for at least 2 weeks before beginning treatment with riluzole. All participants had unremarkable screening laboratory evaluations, including urine toxicology. At screening, the patients’ mean score on the Hamilton Anxiety Rating Scale (HAM-A) was 21.2 (SD=3.1).
Before riluzole treatment was initiated at the baseline visit (approximately 1–2 weeks after screening), psychometric evaluations and urine toxicology were repeated. The first dose of riluzole was administered at 50 mg/day, and subsequent doses were fixed at 50 mg b.i.d. for the remainder of the study. Patients were seen each week for the first month and biweekly for the second month for rating scales, medication compliance check, and adverse events monitoring. No psychotherapy or additional psychotropic medications were allowed during the study period.
At endpoint (week 8), patients underwent full psychiatric and medical assessment. A trained independent evaluator who was aware of patients’ medication status but masked to visit week administered rating scales with the study psychiatrist (S.J.M.). Response was defined as a 50% decrease in HAM-A score from baseline, and remission was defined as a HAM-A score of ≤7. Secondary outcome measures were the 24-item Hamilton Depression Rating Scale (HAM-D), the self-report Anxiety Sensitivity Index
(9), and, for patients with comorbid panic, the Panic Disorder Severity Scale
(10).
All statistical tests were two-tailed, and the significance level was set at p≤0.05 throughout. After complete description of the study to the subjects, written informed consent was obtained. The study was approved by the New York State Psychiatric Institute Institutional Review Board.
Results
A total of 87 subjects were screened for this study; the final group included 18 eligible patients. Of the 18 patients who took at least one dose of riluzole, 15 (83%) completed the 8-week trial. Two patients dropped out in the first week due to adverse events (dizziness and nausea in one patient, cognitive slowing in the second), and a third patient was taken out of the study because of a protocol violation. Because all three dropouts occurred before the first postbaseline assessment, no analyses of their efficacy data were conducted.
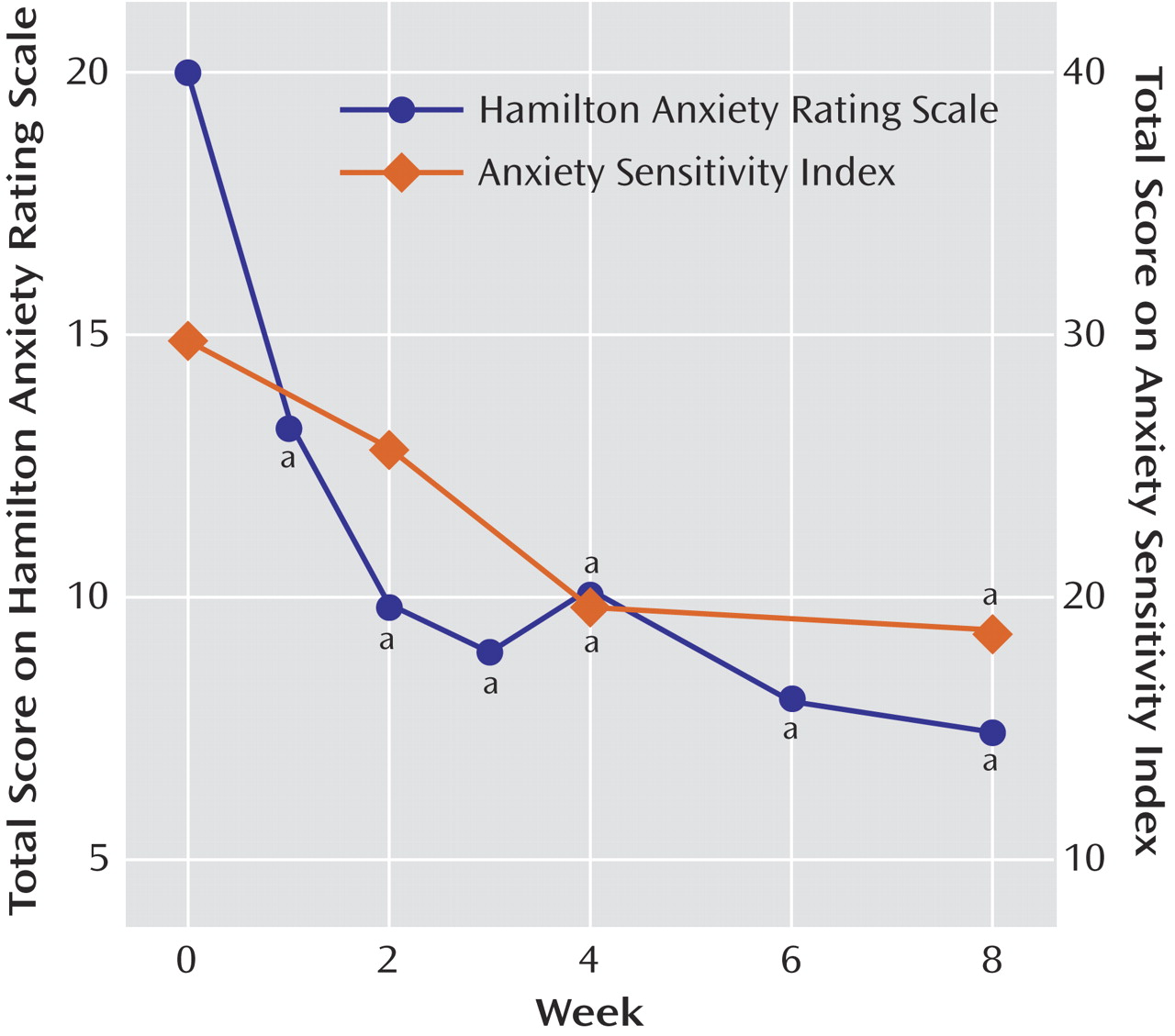
Twelve of the patients had responded positively to the drug by week 8; these patients represented 67% of all patients (intent-to-treat group) and 80% of the patients who completed the trial. Eight patients were in remission at the end of 8 weeks, representing 44% of all patients and 53% of those who completed the trial. The mean HAM-A score of the 15 patients who completed the trial decreased from 20.0 (SD=3.4) at baseline to 7.5 (SD=5.3) at week 8 (
Figure 1). Mean scores on the psychic subscale of the HAM-A improved from 13.0 (SD=2.5) to 5.0 (SD=4.0) (paired t=7.42, df=14, p<0.001), and mean scores on the somatic subscale improved from 5.0 (SD=4.0) to 2.5 (SD=1.6) (paired t=5.60, df=14, p<0.001). Median time to response was 2.5 weeks (range=1–6). Analysis of variance for patients who completed the trial indicated significant improvement in HAM-A score across the duration of the trial (F=17.34, df=1, 7, p<0.0001) (
Figure 1). Dunnett’s post hoc analysis showed that improvement occurred after 1 week of treatment and persisted throughout the study (p<0.05 compared with baseline at each time point).
Mean Anxiety Sensitivity Index scores decreased from 29.8 (SD=10.4) to 19.4 (SD=8.9) (paired t=4.40, df=14, p=0.001) (
Figure 1), and HAM-D scores decreased from a mean of 15.3 (SD=4.4) to a mean of 9.5 (SD=7.3) at endpoint (paired t=3.63, df=14, p=0.003). Age, gender, psychiatric comorbidity (including presence of panic attacks or disorder), or previous pharmacotherapy did not differentiate patients whose anxiety did or did not remit. Of the seven patients with comorbid panic disorder, two discontinued because of side effects, three were remitters according to HAM-A criteria, and two were nonremitters by HAM-A criteria. All five completers experienced marked reductions in panic symptoms, from a baseline Panic Disorder Severity Scale mean of 10.4 (SD=4.92) to an endpoint mean of 1.40 (SD=1.14) (paired t=4.44, df=4, p=0.01).
The most common adverse events during the trial were insomnia/sleep disturbance (three patients [22%]), nausea/abdominal discomfort (three patients [22%]), sedation (two patients [11%]), and dry mouth (two patients [11%]). No serious adverse events were noted. One patient who completed the trial required a dose reduction of riluzole to 50 mg/day for the final 4 weeks due to sedation. Three patients exhibited a transient increase in alanine aminotransferase ranging from 1.1 to 1.8 times the upper normal limit in week 4 (N=1) or week 8 (N=2). The aminotransferase values of the patient with the earliest detected elevation normalized within 2 weeks while receiving riluzole and remained normal at endpoint; for the remaining two patients, aminotransferase values normalized after discontinuation of riluzole. No patient exhibited symptoms of hepatic toxicity.
Discussion
In this open-label pilot study, riluzole at a fixed dose of 100 mg/day was associated with rapid and sustained anxiolytic effects in patients with generalized anxiety disorder, with favorable tolerability. We did not observe substantial elevations of liver function test values in this patient group, but data from other clinical trials
(11) suggest that liver function monitoring is warranted. Clinician-rated psychic and somatic aspects of anxiety as well as patient-reported measures of anxiety sensitivity were significantly improved. Remission and response rates exceeded those achieved by other nonbenzodiazepine medications
(2,
3) and may have been even greater at the higher doses used by Zarate et al.
(7) for treatment-resistant depression. Important limitations of the current study include the small number of subjects and lack of placebo control; the outcome might differ in a controlled design.
Although our results must be considered preliminary because of the open-label design, they offer further evidence in support of the role of the glutamatergic system in anxiety disorders and the anxiolytic properties of antiglutamatergic agents. Riluzole was recently observed to have beneficial effects in a patient with obsessive-compulsive disorder
(12), and additional studies in other anxiety and mood disorders are ongoing. It is notable that Panic Disorder Severity Scale scores uniformly and significantly decreased not only in patients whose anxiety remitted but also in those whose anxiety did not remit. This suggests potential efficacy of riluzole in panic disorder. Larger controlled trials are warranted in generalized anxiety disorder and related anxiety disorders such as panic disorder and social anxiety disorder.