Selective serotonin reuptake inhibitors (SSRIs) have been shown to be efficacious in children and adolescents for the treatment of depression
(1–
3). The presumed mechanism of action for the therapeutic effect of SSRIs is blockade of the serotonin reuptake transporter on presynaptic neurons, with the subsequent increase in transmission by serotonergic neurons. Human platelets have a serotonin reuptake transporter that is identical to the one in the brain
(4), thus the platelet transporter may be used as a surrogate marker for the effect of SSRIs on serotonergic neurons.
Previous positron emission tomography data have indicated that adults taking 20 mg of paroxetine or 20 mg of citalopram had a 77% mean occupancy of the serotonin transporter in the brain
(5). It has also been observed that at the typical minimum therapeutic doses of SSRIs in adults (e.g., 20 mg of fluoxetine or paroxetine, 50 mg of sertraline, 40 mg of citalopram), the mean serotonin reuptake inhibition in patient platelets was 60%–80%
(6). However, the extent of platelet serotonin reuptake inhibition has not been quantified during SSRI treatment in adolescents and has not been directly related to clinical response in this population or in adults.
This study determined the change in platelet serotonin reuptake in 23 adolescents with depressive symptoms who participated in a 14–28-day pharmacokinetic study of citalopram (20 mg/day), paroxetine (20 mg/day), or sertraline (50 mg/day). The relation of serotonin reuptake inhibition to clinical response was also examined.
Method
Subjects were participants in pediatric SSRI pharmacokinetic studies that were approved by the University of Pittsburgh Institutional Review Board
(7). They were referred to the study by their attending child psychiatrist for initiation of SSRI treatment. The subjects’ parents or guardians as well as those subjects aged 14 years or older provided written informed consent prior to initiation of any study procedures. Verbal assent was obtained from children 13 years of age or younger. Subjects had a pretreatment interview that included the depression section of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS), present episode version
(8). Subjects also completed the Beck Depression Inventory
(9) or Children’s Depression Inventory
(10) and had a clinical interview with a board-certified child psychiatrist (D.A.A.) to confirm the appropriateness of SSRI treatment. Subjects were included in this analysis if they met DSM-IV criteria for a current depressive diagnosis. Of the 23 subjects who were depressed at intake, 13 had major depressive disorder, two had dysthymic disorder, and eight had depressive disorder not otherwise specified. There were 10 male and 13 female subjects, with ages ranging from 13.1 to 17.7 years (mean=15.1, SD=1.2). Seventeen subjects were white, five were African American, and one was Asian American. All subjects were physically healthy and had normal physical examination results at study entry.
On the morning before starting the first dose of the SSRI, subjects had blood drawn for analysis of pretreatment platelet serotonin reuptake inhibition. They then took a daily morning dose of citalopram, 20 mg (N=13); sertraline, 50 mg (N=8); or paroxetine, 20 mg (N=2). After 14–28 days (mean=16.2) of treatment, subjects returned in the morning to have blood drawn before taking their morning dose to measure posttreatment platelet serotonin reuptake. Repeat assessments with the K-SADS depression interview and either the Beck Depression Inventory or Children’s Depression Inventory were performed at that time. Subjects returned to their referring psychiatrist for follow-up treatment. Subjects did not take other psychotropic medications for at least 2 weeks before and during the study. All had negative urine drug screen results before entering the protocol.
Clinical response of depressive symptoms during the 14–28-day interval between platelet reuptake measurements was rated retrospectively by the lead author (D.A.A.) using the Clinical Global Impression (CGI) improvement subscale. The ratings were based on the K-SADS depression interviews, Beck Depression Inventory/Children’s Depression Inventory self-reports, and clinical progress notes. The CGI was performed blind to the results of the platelet data.
The method of Tuomisto and colleagues
(11) was used as the procedure for the platelet serotonin reuptake assay but was modified by reducing the blood volume collected to 20 ml. Platelet concentration was determined by a Coulter count, and samples were diluted to a platelet concentration of 200,000×10
3/μl. Serotonin uptake was measured at nine standard concentrations of [
3H]serotonin within 2.5 hours of obtaining the sample from the subject. The interday coefficient of variation of the assay ranged from 9.1% to 14.6%. For the calculation of maximum velocity (V
max) of serotonin uptake into the platelet, the raw data (from scintillation counter measurements at each of the nine concentrations) was converted into a V versus serotonin plot that was fit to the Michaelis-Menten equation by nonlinear regression using Enzfitter version 2.0.8 (Biosoft, Ferguson, Mo.). V
max was measured in picomoles of serotonin/10
7 platelets/5 minutes. Platelet serotonin reuptake inhibition was determined by the change in V
max from pre- to posttreatment and was expressed as the percentage change from the baseline V
max for the primary analysis. SPSS version 11.5 was used for the nonparametric correlation analysis and for an ordinal regression analysis using a logit link function (SPSS, Inc., Chicago).
Conclusions
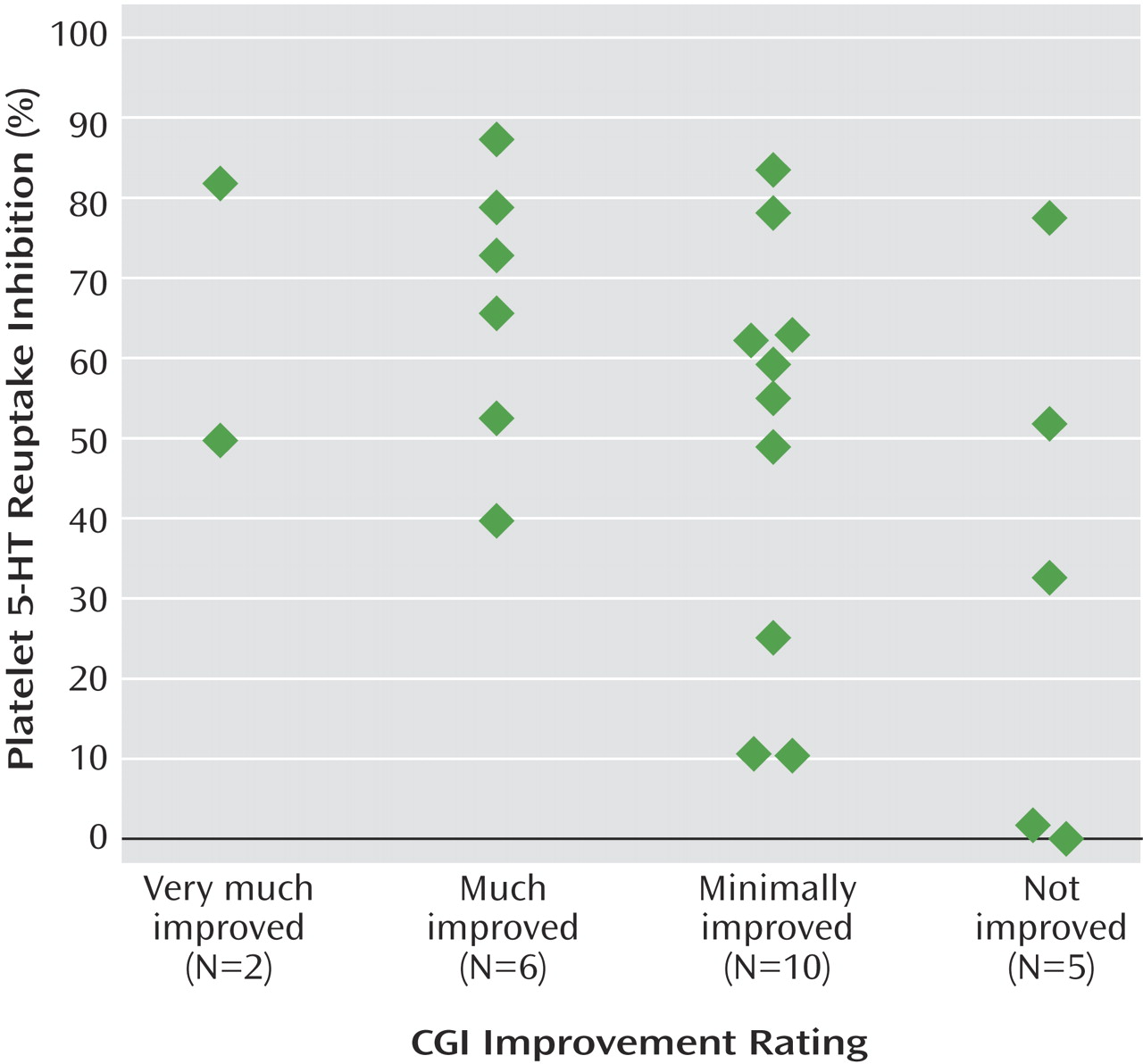
To our knowledge, this is the first study in adolescents that quantifies the pharmacodynamic effect of SSRIs on a biological marker and shows a relation of the marker to clinical response. This is a preliminary study and should be viewed with the following limitations in mind. The study sample was small and was heterogeneous in the intensity of depressive symptoms at intake. Final clinical response was determined retrospectively, although it was based on data that was obtained prospectively and conducted blind to the results of the platelet assays. The duration of SSRI treatment prior to measuring response was relatively short, so some subjects may not have had time to exhibit a complete response to the medication. Consequently, a substantial proportion of subjects were rated as having minimal improvement, and platelet results were widely distributed in this group. However, the effect size of the difference in platelet serotonin reuptake inhibition between subjects who showed unequivocal clinical improvement (CGI rating of 1 or 2; mean inhibition=66% [SD=17]) and those who had absolutely no response (CGI rating of 4; mean inhibition=33% [SD=33]) was large (d=1.4). Despite the aforementioned limitations, the results demonstrate that platelet serotonin reuptake inhibition may be an appropriate surrogate biological marker of the pharmacodynamic activity of SSRIs.
The development of appropriate surrogate biomarkers of treatment response to SSRIs in youth can have potential use clinically as well as have implications for whether this class of medication has therapeutic effects in this patient population. SSRI blood levels have not been shown to correlate with clinical response in numerous studies of depression in adults
(12). As it can take several weeks at the proper dose of an SSRI to show improvement in depressive symptoms, a biological surrogate marker of response that can be measured early in treatment has the potential to substantially reduce titration time to the proper dose.
The efficacy of SSRIs for depression in the pediatric population has recently been called into question. A recently issued FDA advisory stated that despite submissions of randomized controlled trials of sertraline, paroxetine, citalopram, venlafaxine (a serotonin-norepinephrine reuptake inhibitor), and fluoxetine for pediatric depression, only fluoxetine had sufficient evidence to establish effectiveness for the treatment of pediatric depression
(13). Therefore, data that clinical improvement of depressive symptoms in adolescents is correlated with changes in serotonin reuptake add indirect evidence that SSRIs may indeed be helpful for this patient population.