For the complex disorder of attention deficit hyperactivity disorder (ADHD), one challenge is to elucidate the individual differences in developmental pathways leading to various ADHD outcomes. Many researchers are trying to understand how genetic liability interacts with environment in the development of ADHD and clinical variability
(1–
5). Among our first attempts to illuminate the pathway from genotype to phenotype is an investigation of the relationship between genes, temperament, and ADHD outcome.
The dopamine D4 receptor (DRD4) gene has consistently been widely investigated in the quest to discover the genetic underpinnings of ADHD, with the 48-base pair (bp) variant at the focus of most studies. Complementing our own literature review
(6–
21) in 2001, Faraone et al.
(22) conducted a meta-analysis of 14 case/control and family-based association studies that investigated the 7-repeat allele of the DRD4 48-bp variant and ADHD. In the meta-analysis, they found significant associations for both family-based and case/control studies (odds ratios of 1.4 and 1.9, respectively). Although to our knowledge no comparable meta-analysis exists for DRD4 and novelty seeking, there are some 23 studies that have investigated the 48-bp repeat variant and novelty seeking, with 52% of these showing positive results
(24–
44).
The results of these studies suggest a possible role of DRD4 in both ADHD and novelty seeking, although the estimated effect size is very small and results remain tenuous because of the large number of studies reporting negative findings. Three factors are likely to contribute significantly to the variability in findings, including 1) the polygenic nature of ADHD and temperament and the difficulty in replicating minor gene effects under polygenic inheritance
(45), 2) clinical variability across studies, and 3) the possibility of false positives in light of numerous analyses and nominal p values used to determine significance for many candidate gene investigations. To what extent DRD4 plays a role in the genetic liability to ADHD, novelty seeking, or both thus warrants further investigation. The possible common genetic underpinnings of novelty seeking and ADHD on the basis of individual associations with the same “risk” DNA variant would suggest that they would overlap to some extent at a phenotypic level. However, there is a paucity of research on the relationship of novelty seeking and ADHD and, to our knowledge, no investigation of DRD4 in the context of a joint analysis of the two phenotypes in any one group of subjects.
Temperament has been conceptualized as a genetically influenced building block of personality; it has been shown to be highly heritable and relatively stable across the lifespan
(46). Personality has been suggested to represent a developmental outcome of the interplay of environmental factors with temperament over time
(47–
49). The role of extremes of temperament in the development of psychopathology is well documented in mood and anxiety disorders as well as axis II disorders
(50–
53), yet little is known of the role of temperament in the development of ADHD.
Our interest in investigating the role of temperament in ADHD stems, in part, from the body of data supporting the dopamine D4 receptor gene as a risk factor in ADHD as well as novelty seeking. Novelty seeking is one major category of temperament as defined by Cloninger’s Temperament and Character Inventory
(52). The temperament theories developed by Cloninger implicate dopamine as the neurotransmitter that drives novelty-seeking behavior
(53). Dopamine regulation is suggested as a primary neurotransmitter system involved in ADHD, due in large part to the efficacy of pharmacological treatments targeting dopamine levels in ADHD.
There is only one study, to our knowledge, that has investigated temperament in ADHD. Downey and colleagues
(54) found that ADHD subjects (N=78) scored significantly higher than normal subjects on the temperament scales of novelty seeking and harm avoidance. The investigators used the Tridimensional Personality Questionnaire, a precursor to the more comprehensive temperament scales of the Temperament and Character Inventory. The investigators also found that these ADHD adults had high rates of comorbid depressive disorder, antisocial personality disorder, and alcohol and drug abuse/dependence (47% had a current axis I anxiety or depressive disorder, and 37% had a comorbid conduct disorder or mood disorder as children).
In related studies, Nigg and Hinshaw and their colleagues
(55,
56) examined the contribution of the “big five” personality traits in parents to variability in ADHD outcome in children. In the 1998 study, the authors specifically examined oppositional defiant disorder and conduct disorder as well as ADHD symptoms in adults. It is important to note that these investigators used the NEO-PI instead of the Temperament and Character Inventory. It has been suggested that the NEO-PI reflects personality styles (i.e., the “big five”) rather than temperament; however, the NEO-PI construct of extroversion is thought to correlate with novelty seeking
(55). Nigg and Hinshaw found that parental ratings of personality did predict variability in ADHD comorbidity with oppositional defiant disorder and conduct disorder in children. Children with comorbid conduct disorder or oppositional defiant disorder versus ADHD alone had mothers with lower conscientiousness and fathers with lower agreeableness (meaning greater hostility). This study identified a role of parental temperament on child ADHD outcome without addressing the mode of transmission, be it via genes or environmental influence or a combination of both. The study also did not measure to what extent ADHD in the child was accounted for by his or her own temperament profile
(55). In a subsequent study of adults, Nigg et al.
(56) found a relationship between inattention-disorganization and low conscientiousness and high neuroticism. In addition, they found that hyperactivity-impulsivity and oppositional childhood and adult behaviors were associated with low agreeableness
(56).
This present study tests the hypothesis that novelty seeking and ADHD are associated and that their association is due, in part, to DNA variability at the DRD4 gene. The study group comprised parents with or without a history of ADHD from families that had two or more ADHD-affected children recruited as part of ongoing molecular genetic studies
(6–
8,
57–62). A role of DRD4 and ADHD has previously been demonstrated in this sample for the 48-bp variant and a tandem duplication polymorphism upstream of the dopamine D4 receptor gene
(6,
8) and through a meta-analysis of this sample with other investigators of DRD4 and ADHD
(22).
Method
Cohort
Subjects were 171 parents (87 women and 84 men; mean age=43.4 years [SD=6.2]) from 96 families identified from an ongoing molecular genetic study of ADHD in which families had been ascertained through an ADHD-affected sibling pair. After complete description of the study to the subjects, written informed consent was obtained. Among parents, 56 (33%) had a lifetime history of ADHD, meaning they qualified for a definite or probable lifetime diagnosis of ADHD regardless of current ADHD status. Of these 56 parents, 50% (N=28) showed “persistent” ADHD (i.e., continued to meet criteria into adulthood for either definite or probable ADHD). Among the parents, 87% were Caucasian, 5% were Hispanic, 5% were African American, and 3% were of mixed ancestry. The socioeconomic status (per Hollingshead 1957 rankings) breakdown of the subjects was as follows: class I=11%, class II=33%, class III=19%, class IV=11%, class V=9%, class VI=2%, class VII= 15%. Most (84%) were married, 14% were separated or divorced, 2% had remarried, and 1% never married. About half (51.4%) of the individual parents had completed at least 4 years of college. For a full description of the sample assessment procedures, see Smalley et al.
(58). The 96 families represent a subset of families who were administered the Temperament and Character Inventory
(52), a measure added several years into the data collection protocol. After it was added, all subsequent subjects answered the Temperament and Character Inventory questionnaire and are included in the present study.
Measures
After providing written informed consent approved by the UCLA Institutional Review Board, subjects were assessed with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders
(63). As a supplement to assess ADHD and conduct disorder, the behavioral disorders section of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version
(64) was also administered. Interrater reliabilities for ADHD diagnoses were obtained in a subset of 26 interviews rerated from tapes with weighted kappas of 0.96, 0.95, and 0.95. Diagnosing ADHD in adult samples has been controversial. For this reason, our diagnostic criteria also included a spousal report when possible (161 of the 171 parents [94%] had spousal reports). Otherwise, adults were asked to recall as best as possible their early behavior as well as report their current symptoms.
The Temperament and Character Inventory
(52) is a measure designed to assess differences between individuals in seven basic dimensions of temperament and character. The instrument measures four temperament indices (novelty seeking, harm avoidance, reward dependence, and persistence) and three character traits (self-directedness, cooperativeness, and self-transcendence).
Genotyping
Blood samples were collected from each family member, and DNA was isolated using the Puregene Kit following the manufacturer’s recommendations (Gentra Systems, Minneapolis). A polymorphic region in the DRD4 gene consisting of a variable number of 48-bp repeats was scored by polymerase chain reaction (PCR) with primer flanking the repeat sequence. PCR amplification was performed in 12.5-μl reactions containing 60 ng genomic DNA, 10% DMSO, 400 μM DNTPs, 0.8 μM each primer, 10 mM KCl, 20 mM Tris-HCl (pH 8.8), 10 mM (NH4) 2SO4, 0.1% Triton X-100, and one unit Vent DNA polymerase (New England Biolabs, Beverly, Mass.). The primers used were D4-42 5′ (GCG ACT ACG TGG TCT ACT CG)3′ and D4-42 5′ (ACG ACG CTC ATG GCC TTG) 3′ (Operon, Alameda, Calif.). Using the MJ Research PTC-100 thermal cycler, DNA was denatured at 98°C for 4 minutes, followed by 32 cycles of 94°C (1 minute), 54°C (30 seconds), 72°C (2 minutes), and final extension at 72°C for 7 minutes. Final PCR products were electrophoresed in a 2.5% Nu Sieve agarose gel in 1× TBE buffer for 2.5 hours at 100 volts. The gels were ethidium bromide stained for 30 minutes and destained in dH2O for 1 hour. Alleles were determined by comparison of bands to known molecular weight standards.
Genotypes were coded as 0 or 1, reflecting the absence or presence, respectively, of the putative “risk” allele (the 7-repeat variant). The total number of genotyped subjects was 127 because 44 samples failed to yield sufficient DNA for genotyping after inclusion in other genetic investigations. The genotyping could not be done on these 44 samples because the dilution samples were degraded at the time of this analysis. The mean scores on Temperament and Character Inventory scales and the distribution of ADHD status for these 44 parents unavailable for the molecular analysis were no different from those available for study (data not shown). In the present study group, the genotype was coded as 0 for 85 parents (67%) and 1 for 42 parents (33%). The homozygous and heterozygous groupings of the 7-repeat allele were pooled because of low frequency counts for the former and previous work suggesting at least one allele imposes an increased risk
(58,
62).
Statistical Analysis
The observed scores of the 171 subjects on the 20 subscales of the Temperament and Character Inventory were subjected to an exploratory factor analysis in order to establish the relations between the subscales and the Temperament and Character Inventory factors (i.e., measurement model) in our study group. Following exploratory analyses, we used confirmatory analyses to test the role of temperament, including novelty seeking, on ADHD in the parents. All analyses were carried out using Mplus, version 2.13
(65,
66).
The exploratory analysis serves two goals. First, it allows a determination of the number of factors needed to explain the common variance of the observed subscales. We tested whether the Temperament and Character Inventory actually has a seven-factor structure as seen in other normative and clinical samples
(50). Second, it provides estimates of the factor loadings of each observed subscale on each of the factors, such that we can evaluate whether the Temperament and Character Inventory subscales cluster in the expected way (i.e., all novelty seeking subscales load on a single factor but not on other factors)
(65–
67). On the basis of the resulting pattern of factor loadings, a confirmatory model was developed and fit to the data. The confirmatory factor model was then used to test the role of temperament and DRD4 on lifetime ADHD diagnosis as well as ADHD symptom variability.
Results
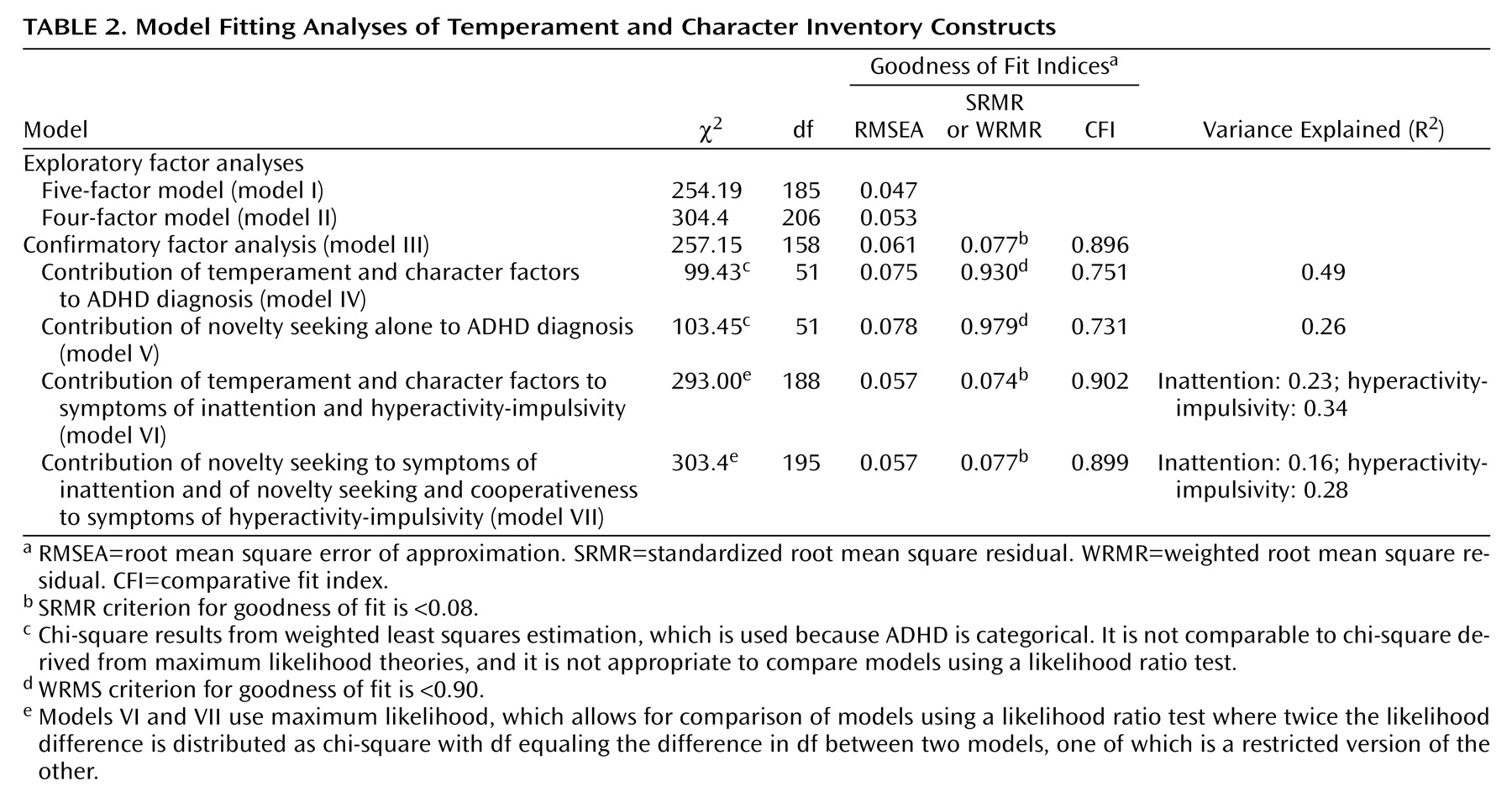
As shown in
Table 1, parents with a history of ADHD had significantly higher scores for novelty seeking and self-transcendence than did the unaffected parents. Overall the parents of ADHD-affected sibling pairs had scores similar to population comparison subjects.
Results of our exploratory factor analyses of the measurement model of the Temperament and Character Inventory are shown in
Table 2.
In exploratory analyses, we varied the number of latent factors from three to eight. In all models, factors were allowed to correlate. The percent of variance (R
2) pertaining to the persistence subscale was below 0.15, and the reward dependence subscales did not cluster on a single factor in any of the models but loaded on several factors simultaneously. Therefore, given the lack of reliability and interpretability for these subscales, they were omitted from further analyses. The resulting five-factor model (model I) provided an acceptable fit to the data. In the context of the five-factor solution, the self-directedness subscale loaded on two separate factors, so the four-factor solution (model II) corresponded better with the known Temperament and Character Inventory structure. Under the four-factor model, the novelty seeking, cooperativeness, and self-transcendence subscales all load on separate factors, while the self-directedness and harm avoidance subscales load on a single bipolar factor, that is, high harm avoidance subscale scores correspond with low self-directedness subscale scores. To retain the four-factor solution but allow for the bipolar factor, we defined in the confirmatory model a factor for self-directedness and one for harm avoidance (i.e., the bipolar factor is modeled as two factors (
Table 2, Model III). Overall, we were largely able to confirm the factor structure of the Temperament and Character Inventory, particularly for novelty seeking, the prime factor of interest.
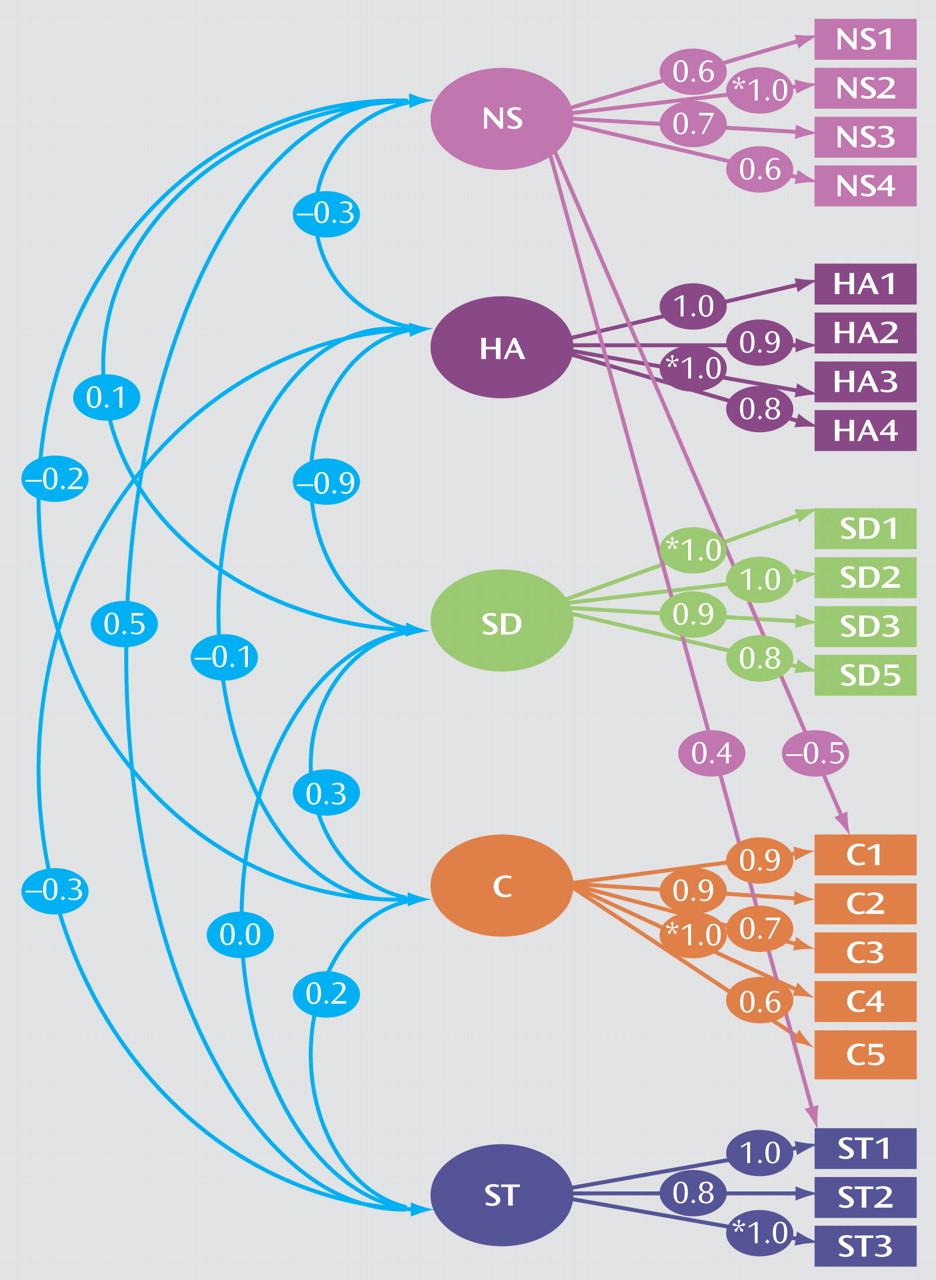
The confirmatory factor analysis measurement model (model III) was specified as the model under which we would perform other analyses with respect to ADHD. This model is depicted in
Figure 1.
Squares represent the observed variables (the Temperament and Character Inventory subscales) whereas circles indicate the unobserved factors. The arrows represent the factor loadings of observed variables on the unobserved factors. The fit of the confirmatory model is acceptable according to commonly used cutoff criteria (e.g., root mean square error of approximation [RMSEA] <0.06, standardized root mean square residual [SRMR] <0.08, weighted root mean square residual [WRMR] <0.9)
(67).
Figure 1 depicts the factor loading estimates. Note that for each factor, one subscale is fixed to 1.0 in order to properly scale the latent factor. In this model, all Temperament and Character Inventory factors are specified to be correlated. The resulting estimated correlations between the novelty seeking, harm avoidance, self-directedness, cooperativeness, and self-transcendence factors are generally low, with the exception of harm avoidance and self-directedness as expected because of their bipolarity being modeled as two correlated factors. We were unable to establish a clear secondary factor structure, featuring two higher-order factors, temperament and character, as proposed by Cloninger et al.
(52).
Using this confirmatory factor model (model III), we tested whether factors underlying temperament and character contribute to lifetime ADHD diagnosis or symptom variability. As shown in model IV (
Table 2), the Temperament and Character Inventory factors explained 49% of the variance in ADHD diagnosis. We tested the role of novelty seeking relative to other latent constructs of temperament and character on ADHD diagnosis (model V). A comparison of models IV and V revealed that novelty seeking was the greatest contributor to ADHD diagnosis, accounting for 26% of variance in the ADHD phenotype. Since novelty seeking contributed to over half of the variance in ADHD and no other Temperament and Character Inventory factor contributed to such a large extent, we selected Model V as the most parsimonious model for subsequent analyses. No other remaining Temperament and Character Inventory factor contributed individually in a significant manner, as reflected by the overall fit of the model with only the novelty seeking factor included.
In addition to testing the role of novelty seeking on ADHD diagnostic status, we examined the role of novelty seeking (and other temperament scales) on individual symptoms of inattention and hyperactivity-impulsivity by using the sum of inattentive items and the sum of hyperactive-impulsive symptoms generated from the psychiatric interview, again based on clinical symptoms reported during the childhood period. As can be seen in
Table 2, novelty seeking and the character dimension cooperativeness accounted for the majority of variability in symptoms of hyperactivity-impulsivity and inattention, as indicated by a comparison of the restricted model for which only novelty seeking and cooperativeness were included (model VII) to the full model in which novelty seeking, harm avoidance, self-directedness, cooperativeness, and self-transcendence were included (model VI) (χ
2diff=10.4, df
diff=7, p=0.17). Under the restricted model, Model VII, novelty seeking and cooperativeness predict hyperactivity-impulsivity with an R
2 estimate of 0.28, while inattention is predicted by novelty seeking, but not cooperativeness, with an R
2 estimate of 0.16.
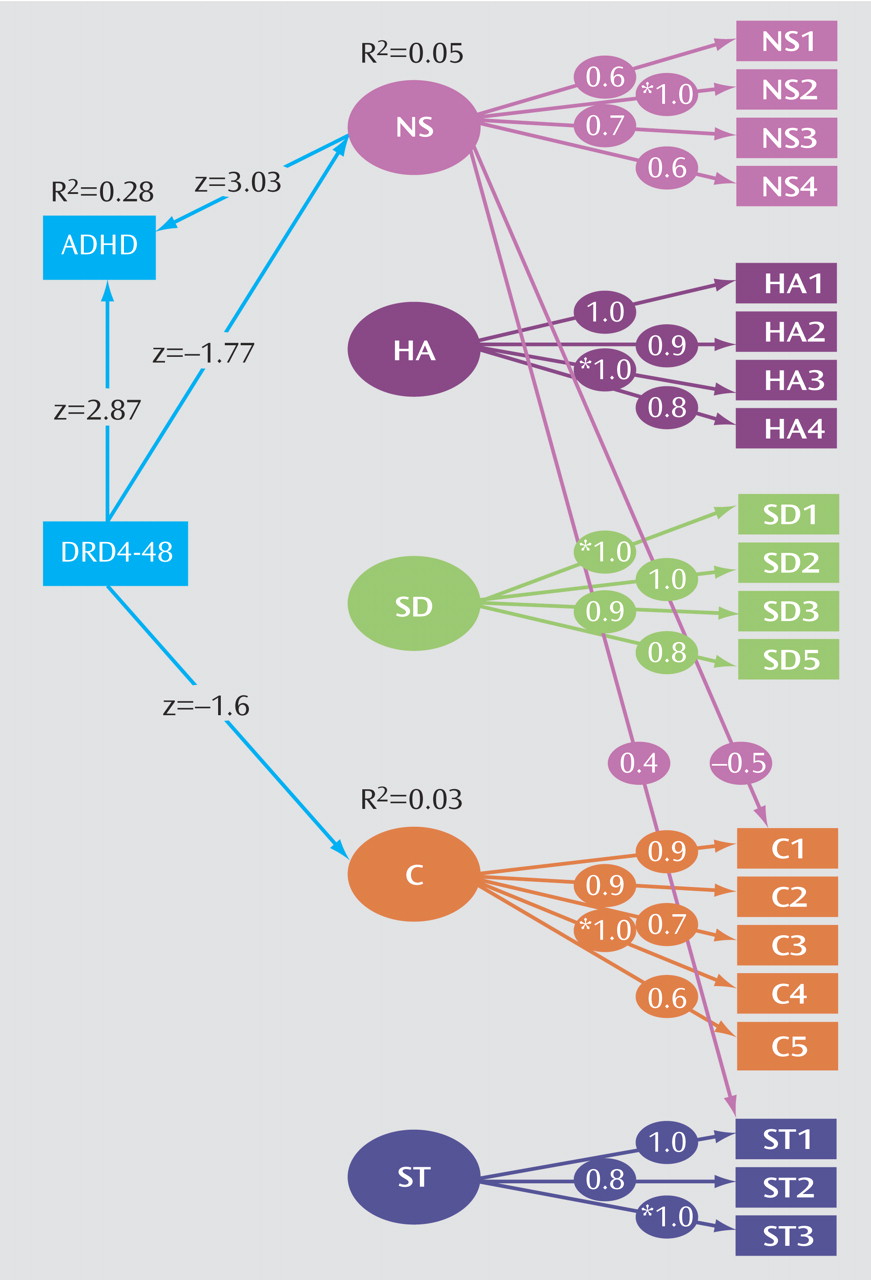
We examined the influence of DRD4 on ADHD diagnosis and symptom sums and the symptoms of hyperactivity-impulsivity and inattention in the subset of individuals who were genotyped for the DRD4 48-bp polymorphism. We tested the unique contribution of DRD4 to ADHD by running a restricted model eliminating the path from novelty seeking to ADHD and comparing it to a full model including novelty seeking. We extended Model V (
Table 2) to include all Temperament and Character Inventory factors again to evaluate DRD4. Dropping the nonsignificant regressions of DRD4 on harm avoidance, self-directedness, and self-transcendence led to a slight increase in model fit due to the increase in parsimony. The model fit well and is shown in
Figure 2.
ADHD is predicted by DRD4 (z=2.87, p=0.004) yet the contribution of DRD4 to novelty seeking is nonsignificant and in the opposite direction (absence of “risk” allele, higher novelty seeking score) than that of ADHD. Eliminating the path from DRD4 to novelty seeking resulted in a negligible change in model fit as reflected by the RMSEA criteria (0.048 to 0.051) and WRMR (0.803 to 0.821). Furthermore, there was a lack of change in variance explained for ADHD (R2=0.28 in both models) with or without DRD4 predicting novelty seeking in the models. The implication of this is that DRD4 and novelty seeking are working more or less independently to influence ADHD, a topic to be examined more in the Discussion section.
Second, we evaluated the role of DRD4 on specific hyperactive-impulsive and inattentive symptoms by extending Models VI and VII in
Table 2. We examined and predicted hyperactivity-impulsivity, inattention, and all Temperament and Character Inventory factors as a function of DRD4. Again, regressions on harm avoidance, self-transcendence, and self-directedness on DRD4 were approximately zero and dropped from the model. The resulting model had a good fit, as reflected by RMSEA=0.041 and comparative fit index=0.941.
The regression weights of DRD4 on novelty seeking and cooperativeness were similar and in the negative direction (i.e., absence of the “risk” allele, higher novelty seeking and cooperativeness scores) while in the positive direction on the hyperactive-impulsive and inattentive symptom sum scores. DRD4 had nonsignificant influences on hyperactivity-impulsivity and inattention, and the only path reaching significance was that of DRD4 on novelty seeking in this model (z=–2.0). Inclusion of DRD4, however, led to an overall modest improved fit of the model relative to one without DRD4 (Model VII,
Table 2) as reflected by the change in RMSEA (0.057 to 0.041) and SRMR (0.077 to 0.073). Excluding all paths from DRD4 except that predicting novelty seeking resulted in no significant change in goodness of fit of the model (χ
2diff=6.48, df
diff=3, p=0.09).
Discussion
The first-order factor structure described by Cloninger’s Temperament and Character Inventory was evident in our dataset. In general, we saw that the observed variables of each construct indeed measured the latent variable as expected. These results strengthen the reliability of the Temperament and Character Inventory as a measure of temperament.
The current study replicates and confirms the findings of Downey et al.
(54) in a sample size twice as large. Specifically, there is a strong role of novelty seeking as a predictive factor of ADHD diagnostic status. While correlation does not imply causality, we modeled novelty seeking and other temperament factors as predictors of ADHD rather than ADHD predicting temperament. This directionality was based on the theoretical and empirical research suggesting that temperamental differences are evident in infancy and early childhood
(45). Temperament, in general, was found to be a major predictor of lifetime ADHD status in parents of ADHD-affected sibling pairs (R
2=0.49), with novelty seeking the primary contributor (R
2=0.26). Similar to Downey and colleagues’ findings, novelty seeking and harm avoidance contribute to ADHD status (although harm avoidance was individually not significant). However, novelty seeking was by far the larger contributor to ADHD. One explanation for the discrepancy for the role of harm avoidance in the Downey study and ours may be that our subjects reflect a more homogeneous (and likely genetic) group of ADHD adults because of the fact that they were ascertained through having at least two ADHD-affected children. Perhaps novelty seeking plays a greater role relative to other temperament scales in familial ADHD than in less familial cases. The temperament scale of self-transcendence was actually more strongly associated with ADHD in our sample than harm avoidance, but neither scale individually reached a level of significance to require inclusion in the most parsimonious model.
We also found a significant role of the character dimension cooperativeness in predicting hyperactive-impulsive symptoms but not ADHD status in the parents. Downey et al. did not have a method to assess this dimension because they used an earlier instrument, the Tridimensional Personality Questionnaire, instead of the more current Temperament and Character Inventory. Since cooperativeness reflects items of character maturity (e.g., “I usually accept other people as they are, even when they are very different from me” and “I cannot have any peace of mind if I treat other people unfairly, even if they are unfair to me”), the relationship of cooperativeness in adulthood to hyperactive-impulsive symptoms in childhood may reflect a continuous developmental trajectory. Conversely, the presence of hyperactive-impulsive symptoms in childhood may contribute to poor development of aspects of maturity, e.g., by interfering with social and emotional regulation. Further work exploring the relationship of this aspect of character development with hyperactive-impulsive symptoms in ADHD is needed.
At the diagnostic level, DRD4 plays a minor but significant role. However, the association of ADHD and novelty seeking was not accounted for by the presence of a risk allele at DRD4. When individual symptom scores (hyperactive-impulsive and inattentive) were evaluated rather than ADHD, DRD4 did not contribute to symptom variability but did have a minor effect on novelty seeking. The pattern of weights from DRD4 to ADHD and novelty seeking suggests that the relationship of DRD4 to ADHD is opposite that of DRD4 to novelty seeking, in contrast to the expectation if the putative “risk” DRD4 variant was accounting for their association. These data strongly suggest that the 7-repeat variant of the 48-bp polymorphism at DRD4 is a genetic variant associated in small part with ADHD (<5%) and possibly novelty seeking (<5%), but that it does not account for the strong phenotypic association of the two traits. To our knowledge, two other studies documented a negative relationship of DRD4 to novelty seeking, but most associations of DRD4 and novelty seeking are in the reverse direction. Gelernter and colleagues
(29) found this sort of negative association specifically in European American women and substance-dependent African American subjects. Malhotra and colleagues
(68) found this negative association specifically in a Finnish population with substance abuse diagnoses. Further work investigating the relationship of DRD4 and novelty seeking in the context of substance dependence or abuse within ADHD samples may clarify the discrepancies observed across studies. DRD4 also contributed a small (and marginally significant) proportion of variance in the character trait cooperativeness, but again in a negative direction (absence of the allele, higher cooperativeness).
The current investigation of DRD4 as a predictor of ADHD or novelty seeking in parents ascertained from multiply affected ADHD families suggests that the strong association of ADHD and novelty seeking observed in the current sample is not due to the small influence of the DRD4 48-bp 7-repeat variant. A minor role of this genetic variant on ADHD is indicated, but its influence pales in comparison to that of the personality construct novelty seeking (25%) in accounting for “liability” to ADHD. It remains impossible to identify whether novelty seeking increases one’s risk for ADHD or whether the presence of ADHD influences the development of novelty-seeking temperament. Assuming that aspects of temperament emerge before the onset of ADHD symptoms, investigations of high novelty-seeking temperament in infancy may prove useful for identifying “at risk” ADHD children. More important, molecular work investigating the genetics of ADHD may benefit from inclusion of novelty seeking as a potential “endophenotype” given its strong association with ADHD and high heritability
(53).
We have furthered the knowledge that parents of children with ADHD, who have a history of ADHD themselves, have significantly high novelty seeking, a temperament factor that may predispose one to ADHD or may be a result of having ADHD. In addition, the parents who have a history of high levels of impulsivity and hyperactivity also have lower scores on the cooperativeness character factor as adults, perhaps suggesting that impulsive behavior leads to reduced development of cooperativeness in adulthood
(52). The present finding has important implications for intervention in ADHD through identifying important parent-based issues. Such parental factors may influence the variability in ADHD symptom persistence, comorbidity, and degree of impairment. This becomes an especially poignant question when considering the immense challenges parents of ADHD children face and that success of interventions may vary as a function of parental characteristics. Every clinician has faced the challenges of some families in which the parents as well as the children are affected with ADHD. An illustrative example of this is a case of a 7-year-old boy, brought in by his parents with the complaints of hyperactivity, difficulty with paying attention at school, and difficulty studying at home all causing major problems. As the evaluation got underway, we discovered the mother’s lifelong struggle with inattention and disorganization. This not only impacted her life but caused her to target this boy all throughout his latency years as being “bad” and “unmanageable since birth when his scream was the loudest.” It became clear as the treatment progressed that this boy, the third of four children, was exceptionally challenging for this mother who had struggled with similar symptoms. This complicated the treatment of the boy in many ways. Psychotherapy for the family was helpful to alleviate damage to the boy’s self-esteem, and proper medication and psychotherapy for the mother helped alleviate her struggle as well. Although the present study does not review the limited data on specific difficulties presented in these cases of both parents and children having ADHD, our work does suggest that further investigation of this issue is important. In light of major efforts underway to best understand how to intervene and influence the development of ADHD and curtail the development of comorbidity and dysfunction in individuals with ADHD, this study may help guide our efforts of treatment development. Targeting character development or development of coping mechanisms in the parents of ADHD children, as well as in the ADHD children themselves, may serve to improve treatment outcomes. This would mean targeted psychotherapy in some cases with parents in addition to any psychopharmacologic interventions.
Limitations of the present study include the possibility that our results cannot generalize to ADHD adults because parents of ADHD-affected sibling pairs may differ. In addition, ADHD diagnoses in these parents were based on retrospective data, and recall bias may affect findings. Furthermore, high novelty-seeking individuals may differentially report themselves as having ADHD. To buffer against this sort of reporting error, we did use observer ratings, clinical evaluations, and spouse reports to ensure the most reliable diagnosing.