Hyperprolactinemia is a frequent and serious side effect of antipsychotic treatment. It has been reported that 48%–93% of premenopausal women and 42%–47% of men taking antipsychotic medications have hyperprolactinemia
(1,
2) . Hyperprolactinemia may cause sexual dysfunction, amenorrhea, infertility, galactorrhea, and osteoporosis
(3) . Studies have reported that 25%–65% of patients with schizophrenia suffer from bone loss after taking antipsychotic drugs
(4 –
5) . Bone fractures in people with schizophrenia taking antipsychotics also occur more frequently than in the nonpsychiatric population
(6) .
Although the introduction of second-generation antipsychotics has reduced the prevalence and severity of hyperprolactinemia, hyperprolactinemia still commonly occurs in patients maintained with haloperidol and many other antipsychotic medications. Outside of the United States, haloperidol is very commonly used as a first-line agent to treat psychosis. Moreover, some second-generation antipsychotic drugs also cause a marked and sustained increase in serum prolactin levels
(7 –
11) .
Generally, three strategies have been recommended for the treatment of this condition: reduction of antipsychotic dose, administration of adjunctive dopamine agonists, such as amantadine or bromocriptine, or discontinuation of current treatment with a switch to a different antipsychotic agent. These strategies, however, can lead to other adverse consequences, such as worsening of psychotic symptoms, which may put the patient at a greater risk for adverse consequences, possibly worse than experiencing hyperprolactinemia itself. Switching to prolactin-sparing second-generation antipsychotics such as olanzapine, quetiapine, or clozapine can be effective for the treatment of hyperprolactinemia
(9,
12) . However, switching to these drugs is not always possible in clinical practice, especially if the patient has responded well to the antipsychotic that produced the hyperprolactinemia. Moreover, these antipsychotics may produce other adverse effects, such as weight gain, diabetes, and cardiac abnormalities.
Aripiprazole is a potent (high-affinity) partial dopamine D
2 agonist, serotonin 5-HT
1A agonist, and 5-HT
2A antagonist. It acts as a functional antagonist at D
2 receptors under hyperdopaminergic conditions but exhibits functional agonist properties under hypodopaminergic conditions
(13) . Because of these unique pharmacological profiles, aripiprazole monotherapy has little effect on and may actually lower prolactin levels in people with prior antipsychotic exposure
(14,
15) . Moreover, aripiprazole is a safe and well-tolerated treatment for schizophrenia
(15,
16) . Thus, based on the pharmacology of aripiprazole and its proven efficacy in schizophrenia, this medication may be an ideal adjunctive treatment for secondary hyperprolactinemia. Although a switch to aripiprazole is an understandable and probably useful strategy, we felt the examination of aripiprazole’s effect as an adjunctive agent was worth examining. If it is clinically effective in reversing haloperidol-induced hyperprolactinemia, rapid adjunctive therapy can be used, with a gradual titration away from haloperidol as a second goal of therapy.
Thus, the primary goal of the present study was to evaluate whether adjunctive aripiprazole, a dopamine partial agonist, improved hyperprolactinemia induced by haloperidol, a potent dopamine blocker, in patients with schizophrenia. Secondary measures were to evaluate whether adjunctive aripiprazole treatment was effective for schizophrenia symptoms and if it contributed to other side effects.
Method
Fifty-six patients with a confirmed DSM-IV diagnosis of schizophrenia were enrolled in an 8-week randomized, double-blind, placebo-controlled study. The inclusion criteria included the following: men and women, ages 18 to 45, clinically stable, who had been treated with haloperidol monotherapy and were taking the same dosage of haloperidol for at least 3 months. Other eligibility requirements included the presence of hyperprolactinemia, no past history of drug or alcohol abuse, and no medical and/or neurological illness.
Aripiprazole was dosed at 15 mg/day for the first 4 weeks, then increased to 30 mg/day for the following 4 weeks if it was clinically tolerated. The study medications were given once or twice daily. The haloperidol dose remained fixed throughout the study, and no other medications that could alter prolactin levels were permitted. Lorazepam, as needed for anxiety or insomnia, was permitted. All patients were enrolled in the study after providing written informed consent. This study was approved by the institutional review board at Inje University.
Prolactin levels were measured at 9:00 a.m., 12 hours after the evening dose of haloperidol was taken. The morning dose of medication was administered after blood was drawn. Blood was drawn at baseline and 4 and 8 weeks after random assignment. In women, study enrollment and laboratory draws coincided with each month’s menstruation. For women with irregular menstrual cycles or experiencing ammenorrhea, study enrollment and monthly blood sampling was independent of the menstrual cycle. Serum prolactin levels were measured with electrochemiluminescent immunoassays, with commercial kits for measuring prolactin (Elecsys 2010, Boehringer Mannheim, Indianapolis). Hyperprolactinemia was defined as a serum prolactin level of >24 ng/ml for women and >20 ng/ml for men. Oligomenorrhea was defined as infrequent, irregularly timed episodes of bleeding occurring at intervals of more than 35 days from the previous menstrual cycle, and amenorrhea was defined as the absence of menstruation for three menstrual cycles or 6 months
(17) .
Severity of psychopathology and adverse effects were evaluated with the Brief Psychiatric Rating Scale, the Scale for the Assessment of Negative Symptoms
(18), the Clinical Global Impression (CGI) for severity symptom scale, the Simpson-Angus Rating Scale
(19), the Barnes Akathisia Rating Scale
(20), and the Side Effect Checklist derived from the items of psychic and autonomic categories of the UKU Side Effect Rating Scale
(21) . These tests were performed at baseline and at weeks 1, 2, 4, 6, and 8. Menstrual disturbances and galactorrhea were evaluated with the Prolactin Related Adverse Event Questionnaire
(22) at baseline and weeks 4 and 8.
Serum haloperidol and aripiprazole levels were measured at baseline and weeks 1, 2, 4, and 8. Plasma concentrations of haloperidol, reduced haloperidol, and aripiprazole were determined by high-performance liquid chromatography–tandem mass spectrometry, as previously described
(23 –
25), with modifications. The interassay precision for all analyses was less than 14.3%.
Two-tailed Student’s t and chi-square tests were used to characterize differences in demographic and clinical variables between the aripiprazole and placebo groups. Repeated measures analysis of variance (ANOVA) was used to evaluate the effect of time and the time-by-group interaction for prolactin levels, clinical rating scores, and the plasma level of drugs. Student’s t test was used to assess differences in prolactin level and clinical rating scores between groups at each time point. Pearson correlation coefficients were used to evaluate the correlation of prolactin levels with drug levels and Simpson-Angus Rating Scale and Scale for the Assessment of Negative Symptoms scores. An alpha level of <0.05 was considered statistically significant, and all tests were two-tailed.
Results
Demographic and Clinical Characteristics
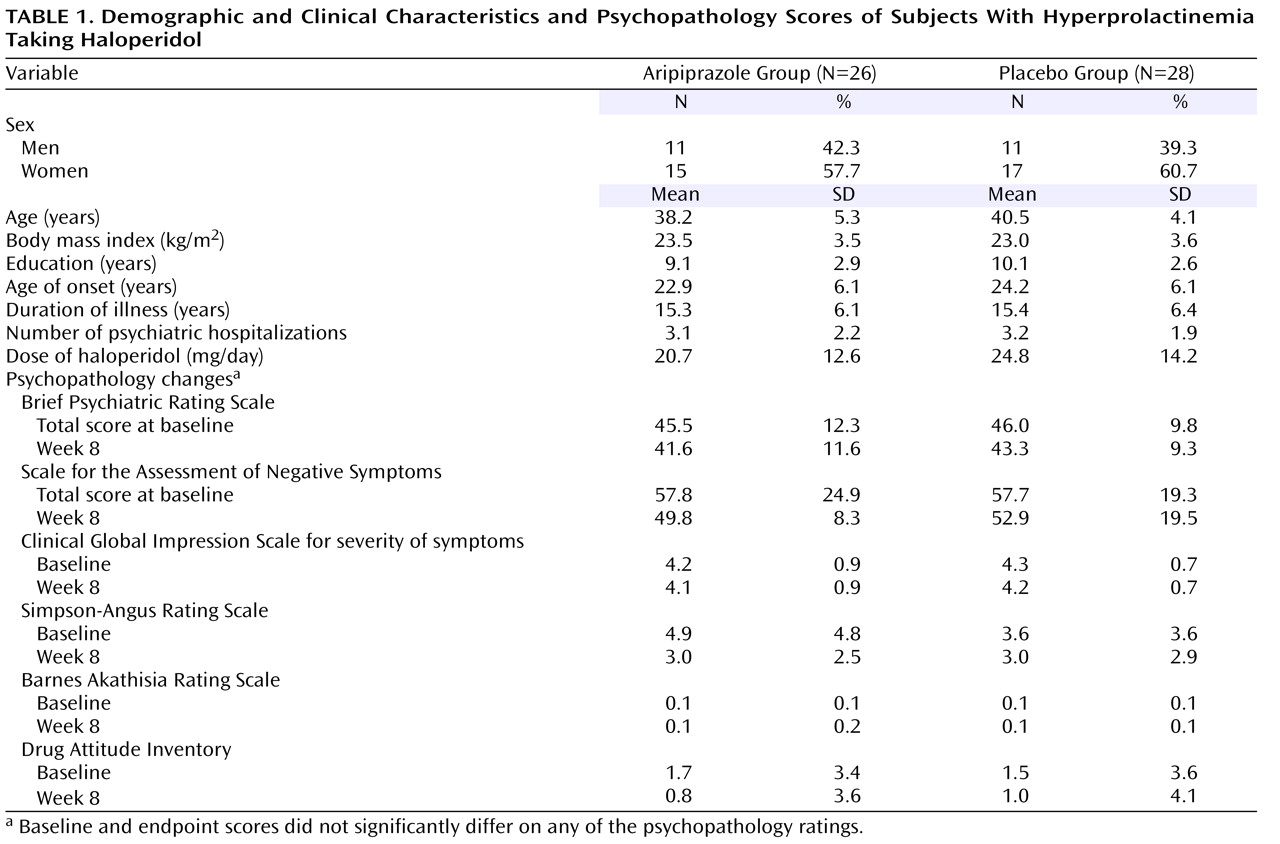
Fifty-six patients with schizophrenia were enrolled into this study. Two patients who were assigned to the aripiprazole group were discharged just after random assignment. Thus, 54 patients (26 aripiprazole and 28 placebo) were analyzed. Among the 54 patients, 52 patients (96.2%) completed the study (24 patients in the aripiprazole group and 28 in the placebo group); two patients (one woman and one man) in the aripiprazole group withdrew from the study at week 4 because of aggravation of clinical symptoms. Clinical characteristics did not significantly differ between the aripiprazole and placebo groups (
Table 1 ).
Prolactin Levels
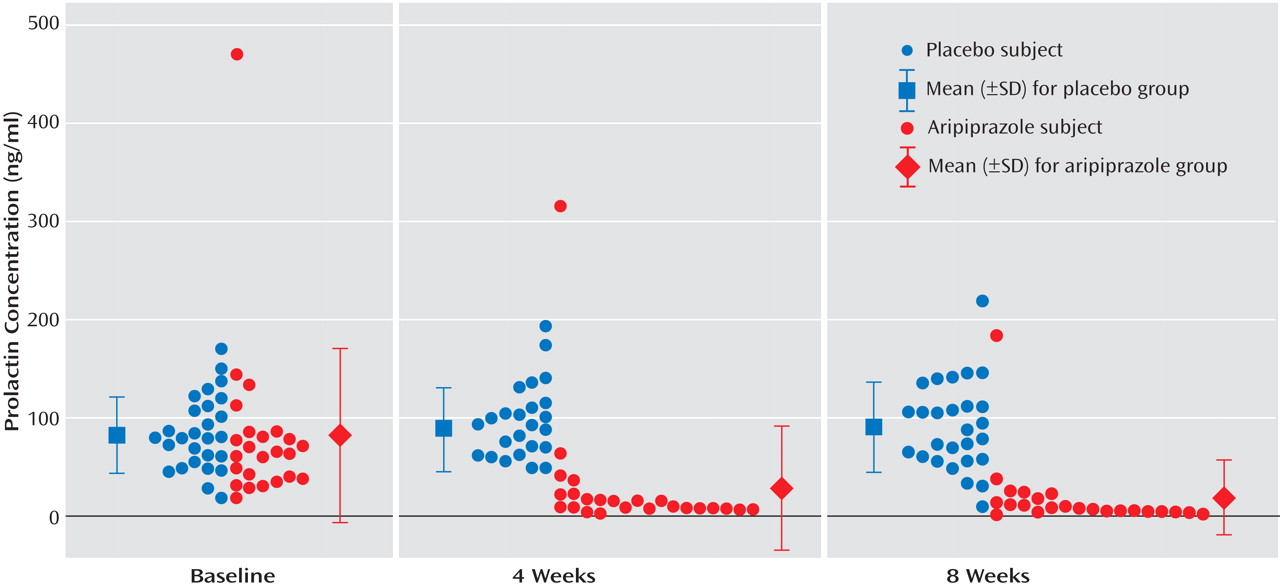
Baseline prolactin levels were not significantly different between the two groups. During the 8-week study, prolactin levels in the aripiprazole group, compared to the placebo group, were significantly lower, demonstrating a significant time effect (F=19.8, df=2, 98, p≤0.0001) and a time-by-group interaction (F=31.8, df=2, 98, p<0.0001) on repeated measures ANOVA (
Figure 1 ). No significant effect was observed in the placebo group over time. The percent decrease in prolactin levels for the aripiprazole-treated patients was 76.5% and 84.2% from baseline at weeks 4 and 8, respectively. In the aripiprazole group, 22 of 26 (84.6%) patients had prolactin levels that improved and were within normal ranges at week 8, whereas one of 28 (3.6%) in the placebo group had normalized prolactin levels (χ
2 =36.2, df=1, p=0.001). When men and women were analyzed separately, the mean baseline prolactin level was significantly higher in women than men (94.6, SD=38.1 ng/ml, versus 56.9 ng/ml, SD=24.3) (t=–3.55, df=51, p=0.0009). However, percent change of prolactin levels from baseline to week 4 and week 8 was not significantly different between male and female patients, and women were represented equally in both groups.
Prolactin-Related Symptoms
Menstrual Symptoms
At baseline, 78.1% (25 of 32) of the female patients were experiencing menstrual disturbances (nine patients with amenorrhea and 16 patients with oligomenorrhea). Mean prolactin levels were significantly higher in patients with menstrual disturbances compared to those having normal menstruation (114.0 ng/ml, SD=94.5, versus 58.6 ng/ml, SD=25.1) (t=–2.95, df=50, p<0.005).
In the aripiprazole group at baseline, 11 of 15 (73.3%) of the female patients were experiencing menstrual disturbances (five had oligomenorrhea and six had ammenorrhea). During the study, 7 of 11 (63.6%) of the female patients having menstrual disturbances regained menstruation (two patients with oligomenorrhea and five patients with amenorrhea). In the placebo group, 14 of 17 (82.3%) of the women had menstrual disturbances at the baseline evaluation (11 had oligomenorrhea and three had amenorrhea). During the study, no patients in the placebo group fully regained menstruation. It was noted that three of the oligomenorrheic patients randomly assigned to placebo had very scanty menstrual periods lasting 1 or 2 days during the study. The mean prolactin levels in patients randomly assigned to aripiprazole who regained menstruation were 94.1 ng/ml, SD=27.9 at baseline, 31.7 ng/ml, SD=24.6 at week 4, and 18.9 ng/ml, SD=14.9 at week 8.
Chest Symptoms
Five female patients were experiencing galactorrhea at baseline: two randomly assigned to aripiprazole and three receiving placebo. At week 8, one of the two patients (50%) receiving aripiprazole no longer complained of signs or symptoms of galactorrhea, whereas all three patients receiving placebo continued to experience galactorrhea. No men were noted to have galactorrhea in the study. No patients were noted to have gynecomastia in this study.
Psychopathology
In a repeated measures ANOVA model, no significant effect over time and time-by-group interaction in total and subscale scores were noted for the BPRS, the Scale for the Assessment of Negative Symptoms and the CGI for severity of symptoms (
Table 1 ). Two patients in the aripriprazole group experienced insomnia, anxiety, and irritability, and both opted for withdrawal from the study at week 4.
Adverse Effects
Overall, no significant changes were noted in the Simpson-Angus Rating Scale and the Barnes Akathisia Rating Scale scores over time in either drug group (
Table 1 ). However, in the aripiprazole group, five of 26 (19%) of the patients improved more than 20% in Simpson-Angus Rating Scale scores, with mean baseline and endpoint scores on the scale being 11.8, SD=5.7, and 4.4, SD=2.1, respectively. Baseline Simpson-Angus Rating Scale scores were significantly higher in patients who had a greater than 20% reduction in score than in those with less than a 20% reduction in score (11.8, SD=5.7, versus 3.2, SD=2.6) (t=5.22, df=24, p=0.001). No patient in either group had worsening of extrapyramidal symptoms or akathisia after adjunctive aripiprazole treatment as measured by these rating scales.
Cumulative side effects in the study for patients randomly assigned to aripiprazole were as follows: insomnia (42%), dry mouth (31%), headache (23%), sedation (12%), and weakness (8%). In the placebo group, the side effects were dry mouth (21%), sedation (18%), and insomnia (18%). Insomnia, dry mouth, and sedation occurred more frequently during treatment with 15 mg/day compared to the last 4 weeks of the study, when aripiprazole patients were receiving 30 mg/day; however, no significant difference in the frequency was noted between doses.
Drug Dosing and Plasma Levels
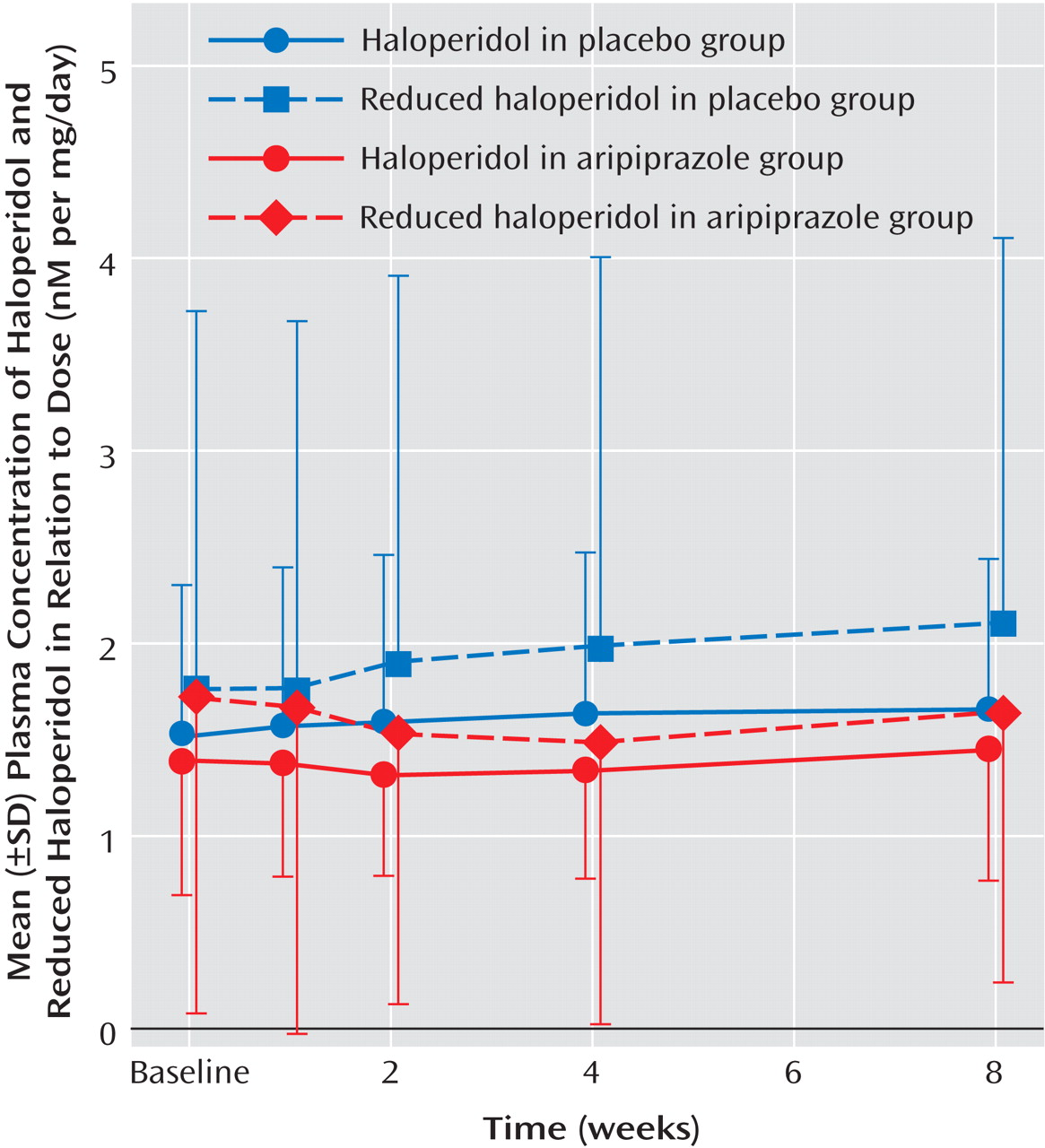
The mean dose of haloperidol was not significantly different between patients randomly assigned to the aripiprazole and placebo groups (20.7 mg/day, SD=12.6, versus 24.8 mg/day, SD=14.2). Baseline plasma levels of haloperidol and reduced haloperidol were not significantly different between the two groups. In a repeated measures ANOVA design, plasma levels of haloperidol and reduced haloperidol did not result in significant time effects and time-by-group interactions after adjunctive treatment of aripiprazole (
Figure 2 ). Plasma levels of aripiprazole were 680.2 nM, SD=405.5 at week 4 and 1233.9 nM, SD=536.5 at week 8 (t=–9.33, df=21, p<0.0001).
Discussion
In the present study, adjunctive aripiprazole treatment successfully alleviated hyperprolactinemia in 84.6% of the patients with schizophrenia and reduced prolactin levels by 84.2% compared to baseline levels. Two patients remained in a hyperprolactinemic state at endpoint (183.6 ng/ml and 37.7 ng/ml); however, their prolactin levels were reduced by 60.9% and 71.7% compared to baseline levels (470.0 ng/ml and 133.4 ng/ml). Coinciding with the improvements in prolactin levels noted with the adjunctive aripiprazole, seven of 11 (63.6%) of the women regained menstruation after adjunctive aripiprazole treatment, whereas no patients did in the placebo group. This finding suggests that adjunctive treatment of aripiprazole is beneficial for the treatment of menstrual disturbances induced by haloperidol. Extremely high prolactin levels have been noted in patients taking dopamine antagonists, particularly in women. While the possibility exists that a pituitary tumor may contribute to these notably high levels and the continued menstrual problems in a few patients, it was found in our study that the patient with the prolactin level at a baseline of 470 ng/ml had a normal magnetic resonance imaging (MRI) scan 3 months before study entry. Thus, it appears that aripiprazole is effective in normalizing prolactin in most patients having hyperprolactinemia taking haloperidol during 8 weeks of treatment, and over 60% of patients having menstrual disturbances can regain their menstrual periods within 2 months after starting aripiprazole treatment. It may take longer than 8 weeks to normalize prolactin levels (not tumor related) for patients experiencing extremely high prolactin levels.
Previous studies have demonstrated the effectiveness of aripiprazole for treating positive and negative symptoms, as well as a safe and tolerable adverse effect profile for both initiating treatment and switching to this antipsychotic from other antipsychotic drugs in patients with schizophrenia
(14 –
16) . In the present study, adjunctive aripiprazole treatment to haloperidol did not produce any significant changes in overall psychopathology as measured by total scores on the BPRS, the Scale for the Assessment of Negative Symptoms, and the CGI Scale for severity of symptoms from baseline to endpoint compared to placebo groups. Current efficacy data for second-generation antipsychotics, including aripiprazole, in the treatment of patients with chronic and/or refractory schizophrenia remains inconclusive. The subjects in our study were chronically ill and clinically stable patients who had a long duration of illness and a history of multiple hospitalizations. These characteristics of our subjects may make it difficult to find further improvements in psychopathology, especially in only 8 weeks. Nevertheless, it is important to note that clinical deterioration or worsening of symptoms with adjunctive aripiprazole treatment for hyperprolactinemia generally does not occur. We did observe a numerically higher percentage of patients with a clinical response in negative symptoms as measured by the Scale for the Assessment of Negative Symptoms in the aripiprazole group compared to the placebo group (30.8% versus 14.3%), although this did not reach statistical significance. This encouraging finding suggests that the effect of aripiprazole as adjunctive treatment for negative symptoms in patients with chronic schizophrenia needs to be formally investigated in future studies. Also, further work is needed regarding the cessation of haloperidol treatment after stabilization with aripiprazole.
No serious adverse events were noted in the study. Two patients experienced symptoms of insomnia, anxiety, and irritability without a change in akathisia as measured by the Barnes Akathisia Rating Scale. These side effects are known to occur in a small proportion of patients given aripiprazole. As expected, the addition of aripiprazole did not worsen extrapyramidal symptoms, and in fact improvements were noted in extrapyramidal side effects, particularly those with high baseline scores on the Simpson-Angus Rating Scale. It is recommended that when one considers adjunctive aripiprazole treatment, attention should be paid to monitoring for symptoms of insomnia, anxiety, and irritability.
Because aripiprazole and haloperidol are metabolized primarily by hepatic enzyme systems, such as cytochrome P450 3A4 and 2D6 enzyme systems
(26,
27), the addition of aripiprazole to haloperidol has the potential to alter plasma concentrations and the metabolism of haloperidol and its metabolite. In this study, plasma concentrations of haloperidol and its major metabolite, reduced haloperidol, were not significantly changed after adjunctive aripiprazole treatment. Aripiprazole has a higher affinity to the dopamine receptor compared to haloperidol
(28) and acts as a partial dopamine agonist or dopamine autoreceptor agonist. Therefore, it is likely that when aripiprazole is administered with haloperidol, aripiprazole may bind to the dopamine receptor more robustly than haloperidol and act as a dopamine receptor agonist in a hypodopaminergic state induced by the long-term use of haloperidol. With these results, we can assume that the change in prolactin level may be due to a pharmacodynamic interaction at dopamine receptors rather than a pharmacokinetic interaction between aripiprazole and haloperidol.
Several limitations of our study should be considered. First, our study did not evaluate whether adjunctive aripiprazole treatment has advantages over aripiprazole alone or in combination with a low dose of haloperidol in the treatment of hyperprolactinemia. However, in this study, the subjects had a high potential risk for relapse due to the chronicity of the population and psychiatric histories. Along with being clinically stable while taking the present dose of haloperidol, these issues make it difficult clinically to discontinue haloperidol treatment. Second, haloperidol dosing may have been higher than ideal for minimizing hyperprolactinemia; however, this dosing was selected by clinicians in real-world treatment settings and considered necessary to treat this population of chronically ill patients. Third, the correlation between aripiprazole dose and the change in prolactin levels may not have been adequately assessed because aripiprazole dosing was started at 15 mg/day and then increased to 30 mg/day. In a previous study, 2 mg/day of aripiprazole acted as a partial dopamine agonist in producing a clinical effect
(29,
30) . It suggests the possibility that doses lower than 15 mg/day of aripiprazole can significantly decrease prolactin levels. The association between aripiprazole dose and prolactin level when used as adjunctive treatment needs to be more fully evaluated. Furthermore, moderate doses of haloperidol as the primary agent should also be evaluated in conjunction with aripiprazole. Fourth, MRIs were not performed as part of study enrollment to rule out pituitary tumors that may have confounded the prolactin results; however, as noted, the one patient with extremely high prolactin levels at baseline had a normal MRI 3 months before study entry. Nonetheless, extremely high prolactin levels should warrant an investigation into other causes before adjunctive aripiprazole treatment to rule out other issues such as a prolactinoma. Finally, the study period of 8 weeks may have been too short to confirm the effect of adjunctive treatment of aripiprazole on menstrual irregularities and disturbances. Thus a longer study (more than three menstrual cycles) may actually show more robust findings. The presence of a perimenopausal state and other hormones, such as estrogen, neither of which was assessed in the present trial, may also mediate menstrual irregularities.
In summary, 8 weeks of adjunctive aripiprazole treatment was effective, safe, and well tolerated in reducing elevated prolactin levels and restoring menstruation in many chronically ill schizophrenia patients stabilized and maintained on haloperidol. Aripiprazole has a higher affinity to D 2 receptors than haloperidol, which is the likely cause of this observation. However, the effect of adjunctive aripiprazole treatment on psychopathology and the long-term adverse effect of this treatment need further study.