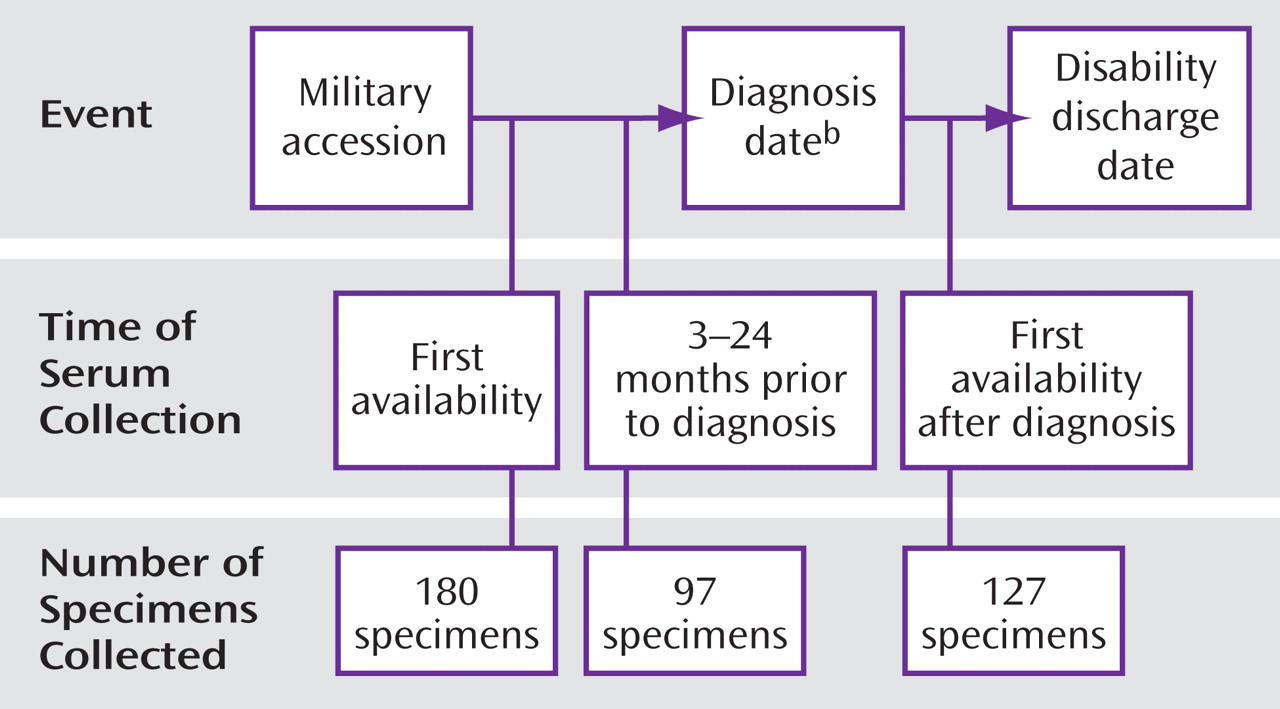
However, notable limitations to these studies are small sample sizes and the reliance on antibody measurements from a single serum sample obtained after diagnosis. Studies of pre-onset serologic and demographic data on large numbers of affected individuals and appropriately matched comparison subjects are difficult to conduct because population-based medical and serologic data are not routinely collected. The U.S. military, however, collects data from all personnel. Serum samples are obtained at military accession and approximately every 2 years thereafter
(18) . Medical encounter data are electronically archived
(19) . This affords a unique opportunity to conduct large, population-based serological analyses on specimens obtained before the diagnosis of disease. We are conducting a series of nested case control studies to explore the hypothesized associations between psychotic disorders and selected infectious agents. In the present hypothesis-generating study, we describe findings from a panel of infectious agents that were analyzed, and we discuss an association between increased levels of antibodies to
T. gondii and subsequent risk of new-onset schizophrenia among military personnel.
Discussion
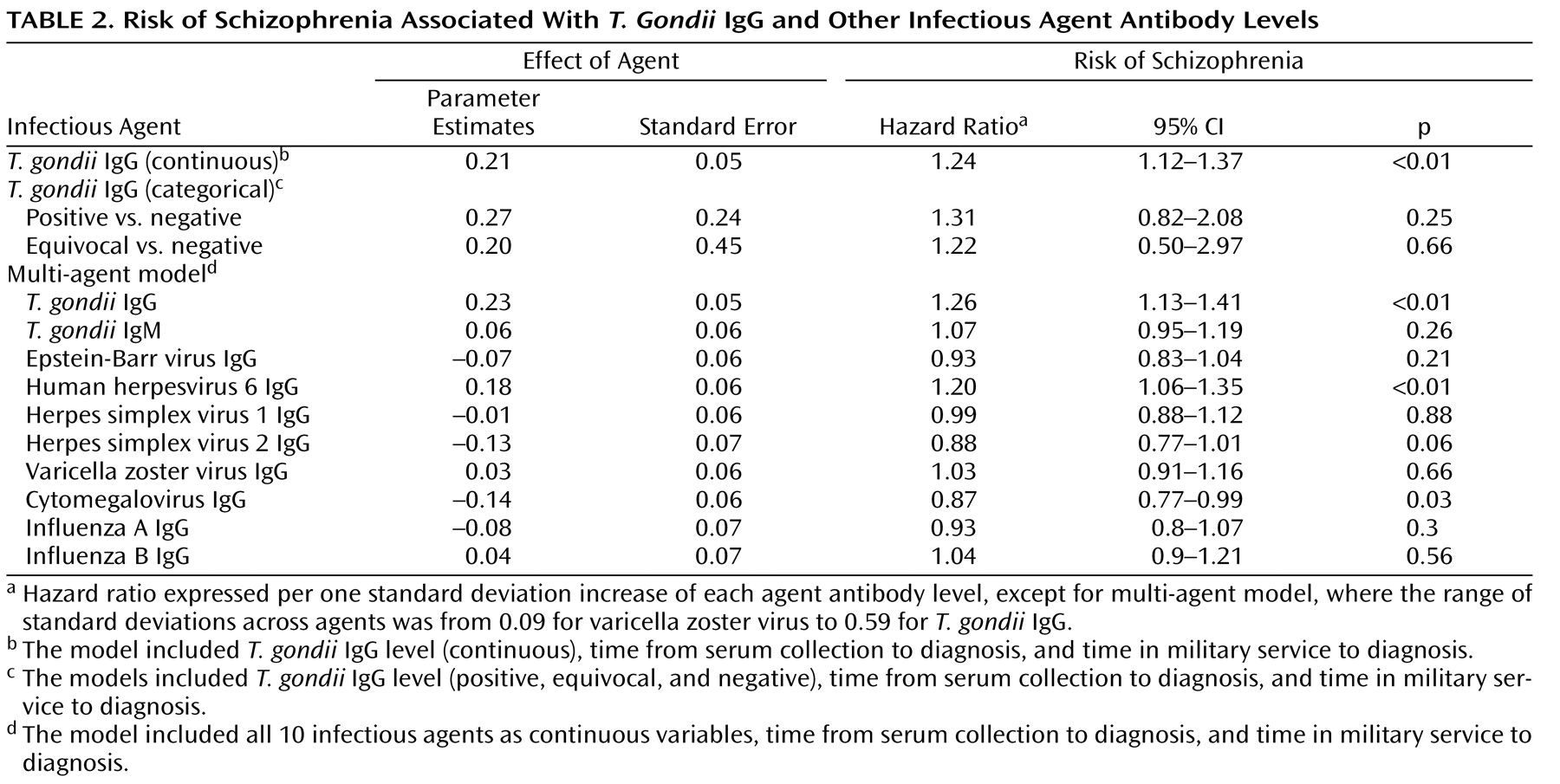
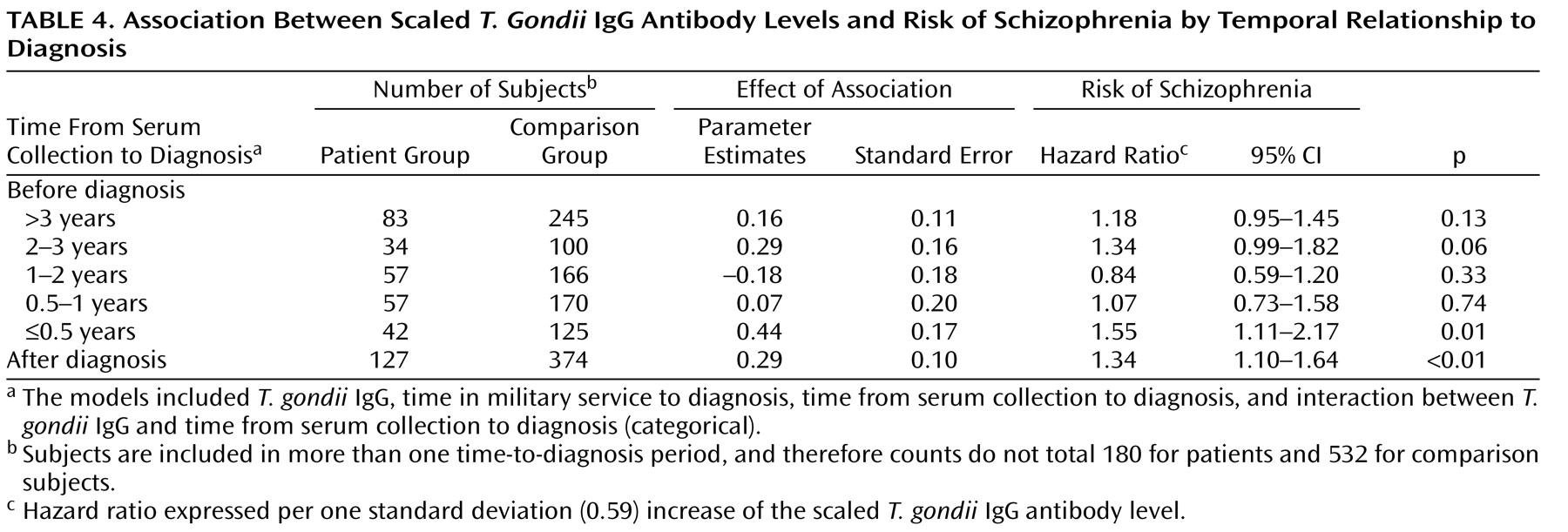
In this study assessing relationships between antibody levels to infectious agents and risk of new-onset schizophrenia, we found a significant association between T. gondii IgG levels and schizophrenia. The addition of other infectious agents to the model did not affect this finding, nor was it substantially modified by sex, age, or race. The T. gondii IgG effect was consistent in nearly all time periods analyzed, both before and after diagnosis.
When T. gondii IgG was studied categorically (positive, equivocal, and negative), results were similar to the continuous analyses (hazard ratio=1.31) but not significant. The lack of significance may be attributable to the small proportion of seropositive specimens (7% for patients; less than 6% for comparison subjects). The hazard ratio for equivocal versus negative was 1.20 (not significant), indicating a positive tendency.
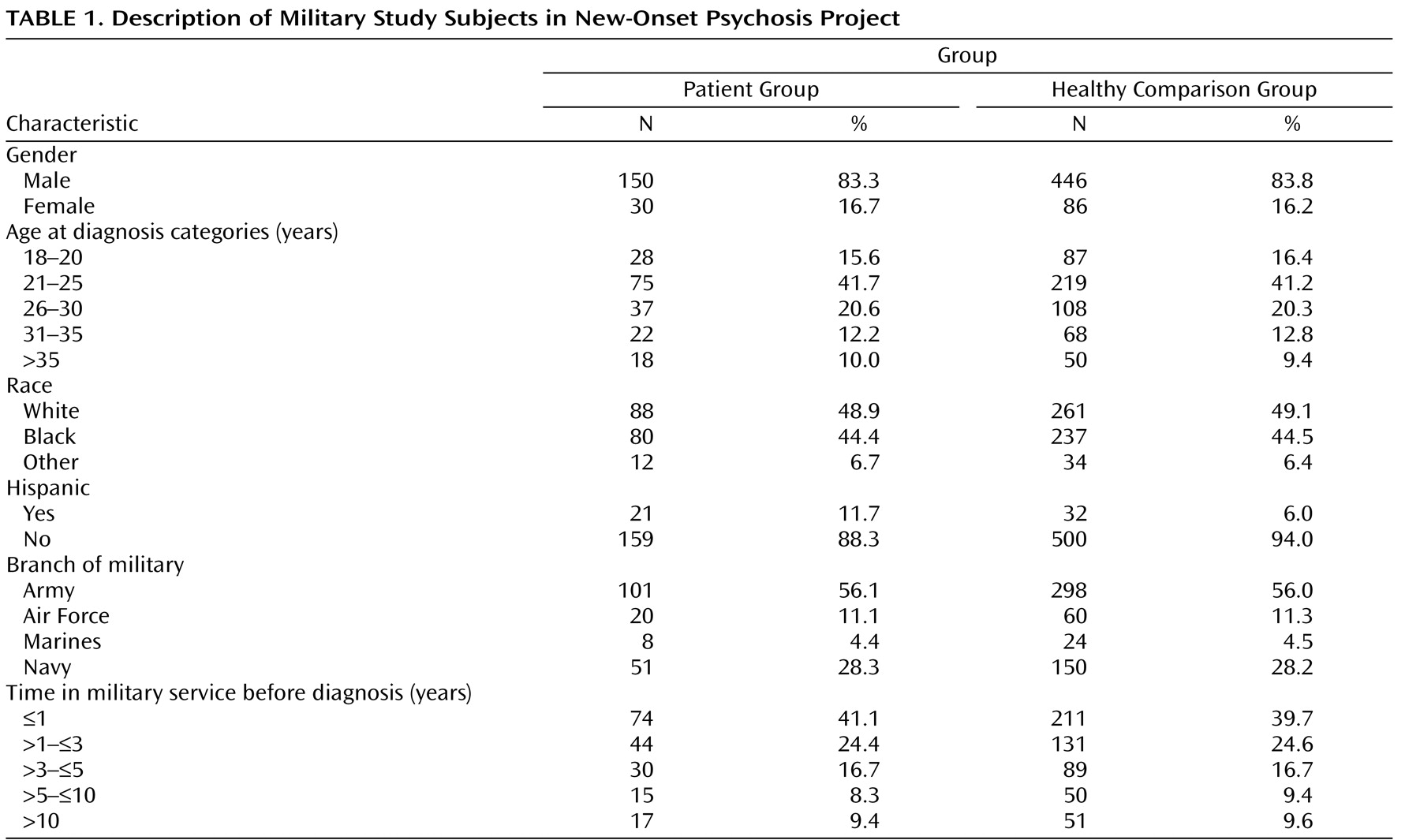
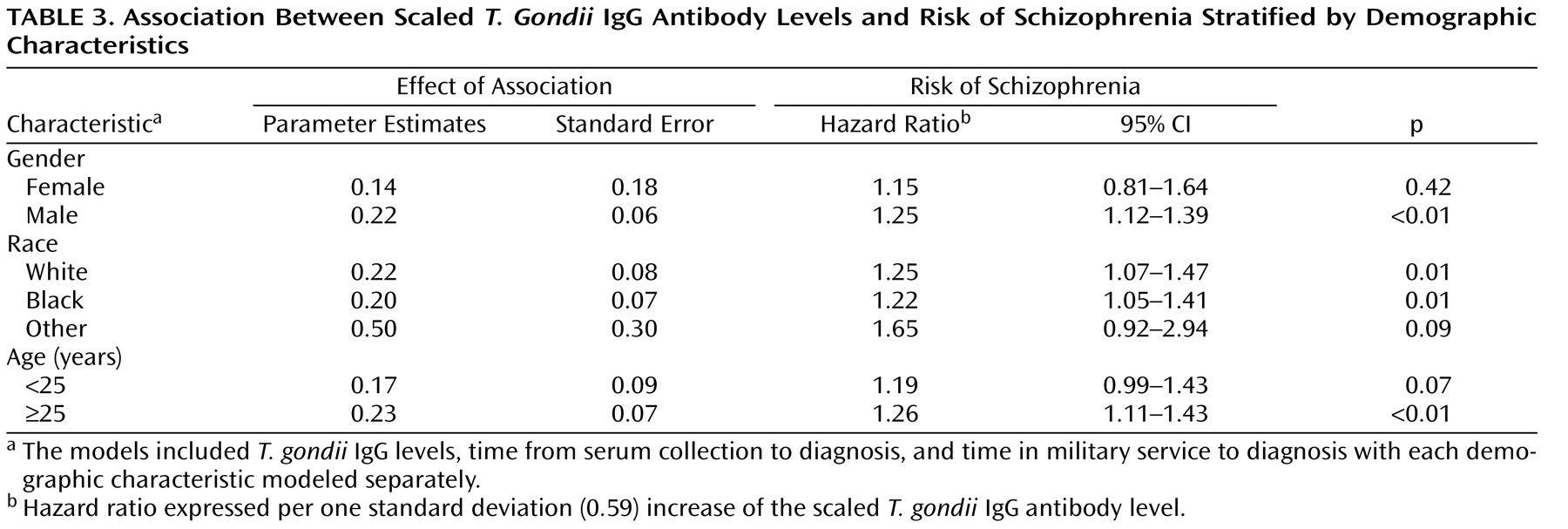
The study cohort size for female and nonwhite subjects was underpowered to assess differences in antibody-level effect. A similar association between T. gondii IgG levels and risk of schizophrenia was observed among these categories. These findings require further exploration in a larger study.
A significant positive association was also observed with HHV-6 IgG levels and schizophrenia, but not with any of the other agents tested. The Herpes family virus antibody levels were included in the model to control for any effect on the relationship between T. gondii antibodies and schizophrenia. A detailed evaluation of the relationship between these viral agents and schizophrenia will be provided in a separate study.
This study has several strengths. Since the study population had serum samples stored in a repository, we were able to retrieve and analyze samples obtained from apparently healthy individuals prior to disease diagnosis. This allowed us to document that elevated levels of Toxoplasma antibody existed prior to symptom onset, making it unlikely that the observed elevations were an artifact of disease-related exposures or a reflection of genetic predisposition to disease. We were also able to obtain multiple specimens per study subject, which will allow detailed longitudinal analysis in future studies. Furthermore, the study design allowed matching of patients and comparison subjects on demographic variables, making it unlikely that the case-control differences observed were related to confounding or other artifactual differences in the two populations.
There are, however, limitations to the current study. Ninety percent of the patients who were potentially eligible for study inclusion were hospitalized in a U.S. Department of Defense medical treatment facility with a mental illness diagnosis. Because of the lack of existing data for most of the study period, we were not able to identify any hospitalizations in non-Department of Defense facilities. According to Hoge et al.
(30), less than 2% of mental health hospitalizations among military service members occur in civilian facilities for emergencies or in remote locations. Therefore, we do not feel that this is an important source of bias or diagnostic misclassification.
In addition, the data are inadequate to evaluate the impact of changes in scaled T. gondii IgG levels over time within individuals in the study population. Because of limitations on serum availability, we could not ensure that every subject was represented in each time period analyzed, resulting in varying distributions of demographic factors across time-to-diagnosis periods (data not shown). Further, we could not obtain equal numbers of specimens per subject. Although variation of the T. gondii effect by gender, race, and age was not significant, marginal differences were observed. The observed temporal effect therefore might also be because of population-specific factors in addition to differential exposure to T. gondii . The model assumed that the log (hazard ratio) is linearly dependent on T. gondii for the continuous predictor. We explored a variety of nonlinear modeling techniques but found our cohort size insufficient to fit them. We expect to find a nonlinear association with a larger cohort size in future analyses, which will increase confidence in the results and allow us to study the residuals, sensitivity, specificity, accuracy, and reliability of the model. Categorical analysis of the data was limited by the very small proportion of T. gondii -positive specimens, resulting in a nonsignificant, although positive, association between risk of schizophrenia and antibody levels.
Finally, identification of subjects for this study relied on diagnostic data obtained through electronic records rather than clinical interviews. Findings from an independent-record review of patients’ medical records, however, demonstrate a greater than 90% verification of diagnoses
(31), indicating that diagnostic misclassification among patients is unlikely to be a major source of bias. Additionally, because a diagnosis of schizophrenia in this population is extremely rare—approximately 0.15 per 1,000 per year since 1990
(19) —it is likely that few, if any, of our comparison subjects were later diagnosed with schizophrenia. Any subsequent misclassification is unlikely to bias the results.
The generalizability of these results to the broader population of individuals in the United States with schizophrenia may be limited by the use of military study subjects. Military populations with schizophrenia differ somewhat from other populations in that they tend to have an older age at onset, most likely because of self-selection and medical evaluation and screening prior to entry into the military. This selection process results in few persons with overt psychosis entering the military. Additionally, the study population differed because all the individuals started out “healthy” and were drawn from a population that is younger than the general U.S. population, with a higher proportion of ethnic minorities and equal access to medical care.
Our findings are consistent with previous studies indicating increased level of antibodies to
T. gondii in individuals with schizophrenia
(15,
17) . However, in previous studies
T. gondii antibodies were measured after diagnosis, raising the possibility that the increased levels of antibodies were the result of disease-related environmental factors
(32) . This study indicates that increased levels of
Toxoplasma antibodies can be found prior to the onset of symptoms and are thus unlikely related to symptom-associated exposures alone.
One challenge to this type of epidemiologic research, involving specimens stored for long and varying periods, is the lack of universally utilized assays and cut-points for ascertaining serum status (positive or negative) and measuring antibody levels. Various strategies have been employed, including arbitrary cutoff selection; categorization by percentile ranges; grouping results as low, intermediate, or high; and analyzing antibody levels as a continuous rather than dichotomous or categorical variable
(15,
16,
24,
33) . In spite of these variations, the consistent finding from most studies is an increased risk of schizophrenia among individuals with high
T. gondii antibody levels, however defined
(14 –
16,
33) .
Given the high prevalence of
T. gondii (approximately 20%) in the United States
(34), it is clear that most infected individuals do not have schizophrenia. The reasons for potential differential reactions to
Toxoplasma infection in patients with schizophrenia is not known but may be related to genetic determinants of host susceptibility
(35,
36), varying degrees of pathogenicity among infecting organisms
(37), or unidentified environmental factors. The timing of infection may be an important differential risk factor, since previous studies indicate that children born to mothers with increased levels of antibodies to
T. gondii are at risk for the development of schizophrenia later in life
(32,
38) . One possible explanation of this finding is that increased antibody levels associated with schizophrenia in adults may be related to immunologic reactivation of infections acquired earlier in life.
The mechanisms by which exposure to
T. gondii might lead to schizophrenia are not known. However, animals experimentally infected with
T. gondii show altered behavior, including abnormal reaction to novel environmental stimuli
(39) . Altered novelty-seeking behavior has also been found in humans with
Toxoplasma antibodies
(40,
41) . Since treatments are available for
Toxoplasma, early detection of
T. gondii infection and initiation of therapy may play a future role in schizophrenia prevention or disease modification.