One therapeutic target identified by the MATRICS is the α
7 -nicotinic acetylcholine receptor. A possible role for the receptor in schizophrenia was first identified in animal models of a sensory gating deficit associated with schizophrenia. Subsequently, evidence for genetic linkage of this sensory gating deficit and for schizophrenia itself was found at the chromosome 15 locus of
CHRNA7, the gene for the α
7 -receptor subunit
(3) . Molecular analysis indicates that the gene’s coding region is intact. Thus, the receptor has normal structure, but the expression is decreased, possibly because of single nucleotide changes in the promoter of the gene
(4) . Postmortem studies show decreased expression of the receptor at its two major sites of expression, the inhibitory interneurons of the hippocampus
(5) and of the nucleus reticularis thalami
(6) . These brain regions are thought to play important roles in the regulation of the brain’s sensitivity to sensory stimuli. Inability to filter out unwanted sensory stimuli is one of the mechanisms of poor attention in schizophrenia
(7) .
Nicotine itself is abused in high doses by many patients with schizophrenia through their heavy cigarette smoking. The a
7 -nicotinic receptor is an order of magnitude less sensitive to nicotine than other nicotinic receptors, and therefore this heavy smoking may be evidence that patients are trying to activate this receptor, perhaps to compensate for its lower than normal expression
(8) . Nicotine was initially observed to reverse the adverse neurocognitive effects of haloperidol
(9) . Positive effects of nicotine on neuropsychological test performance have been observed principally in patients who smoke but have abstained from cigarettes for periods of time ranging from overnight to several weeks. These effects likely represent reversal of withdrawal phenomena
(10 –
13) . Transdermal nicotine in nonsmoking patients had no significant effects on the d′ parameter of the Continuous Performance Test, the measure used for the attention/vigilance domain of the MATRICS battery, but effects were found on other test parameters
(14) . Two studies compared smoking and nonsmoking patients’ responses to nicotine. Among patients who had abstained for only 2 hours, nicotine nasal spay improved delayed recognition but not working memory
(15) . The effect was not seen in nonsmoking patients. We observed the opposite: negative effects of nicotine gum were found on the attention domain of a neuropsychological battery among patients who smoked up until 2 hours before the test, but positive effects were seen in nonsmokers
(16) . These last data raise the possibility that patients’ smoking habits cause tachyphylaxis at nicotinic receptors, and therefore we restricted initial experimental trials of nicotinic agonists to nonsmokers.
The nicotinic agonist 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A) is derived from anabaseine, an alkaloid found in nemertine worms
(17) . The dimethoxybenzylidene derivative is a partial agonist at human α
7 -nicotinic receptors with a half-life of about 2 hours
(18,
19) . An initial proof-of-concept trial in schizophrenia involving single-day administration showed positive cognitive effects, particularly on attention
(20) . The subjects’ antipsychotic drug regimens were maintained, because of the presumption that these drugs are acting through different mechanisms.
On the basis of this initial positive trial, the present phase 2 trial was approved by the U.S. Food and Drug Administration to assess whether cognitive effects would continue during longer-term administration and whether clinical ratings would also change. The doses were those used in the phase 1 trial. The MATRICS battery was chosen because of its recommended use for assessment of drug effects on cognition in schizophrenia
(1,
2) . The Scale for the Assessment of Negative Symptoms (SANS)
(21) and Brief Psychiatric Rating Scale (BPRS)
(22) were used to assess symptoms. As in the initial phase 1 trial, nonsmoking patients, almost all of whom were currently taking antipsychotic drugs, were studied.
Results
Therapeutic Effects
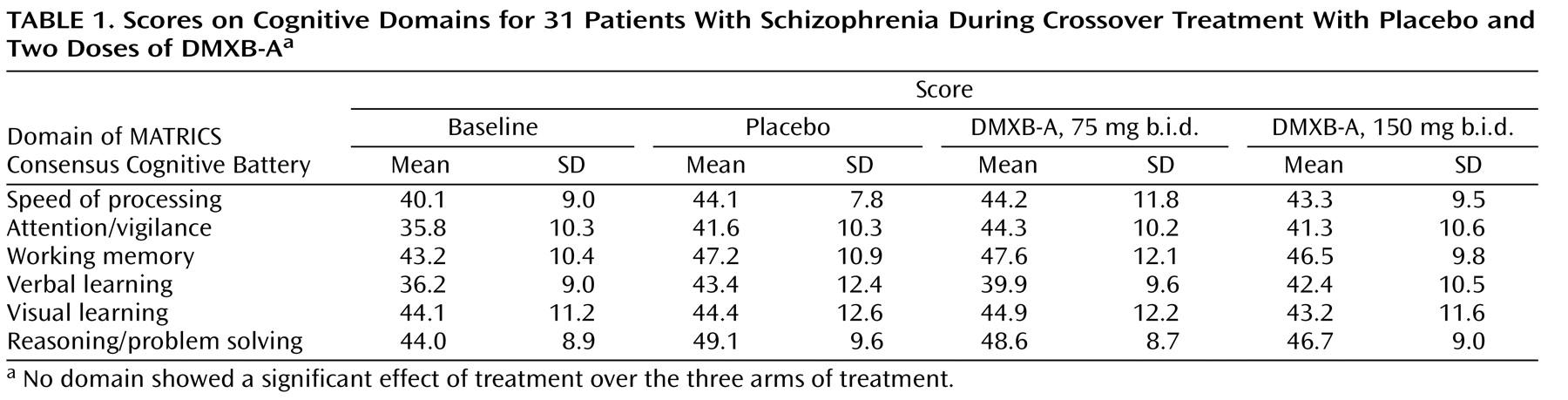
Performance on the six domains of the MATRICS Consensus Cognitive Battery did not differ between either DMXB-A dosage and placebo, which was the primary outcome measurement of the trial (
Table 1 ). Effects of repetition of the tests were observed in several of the domains. For the T score for the speed of processing domain, the effect of encounter number was significant (F=5.96, df=2, 25, p=0.008). The least squares mean difference between week 6, the end of the first arm, and week 16, the end of the third arm, was 4.3 (SD=4.1) (t=3.34, df=26, p=0.002). A nearly significant effect was observed for the T score for the attention/vigilance domain (p=0.08), and changes of similar magnitude, although not significant, were observed for the verbal learning domain T score.
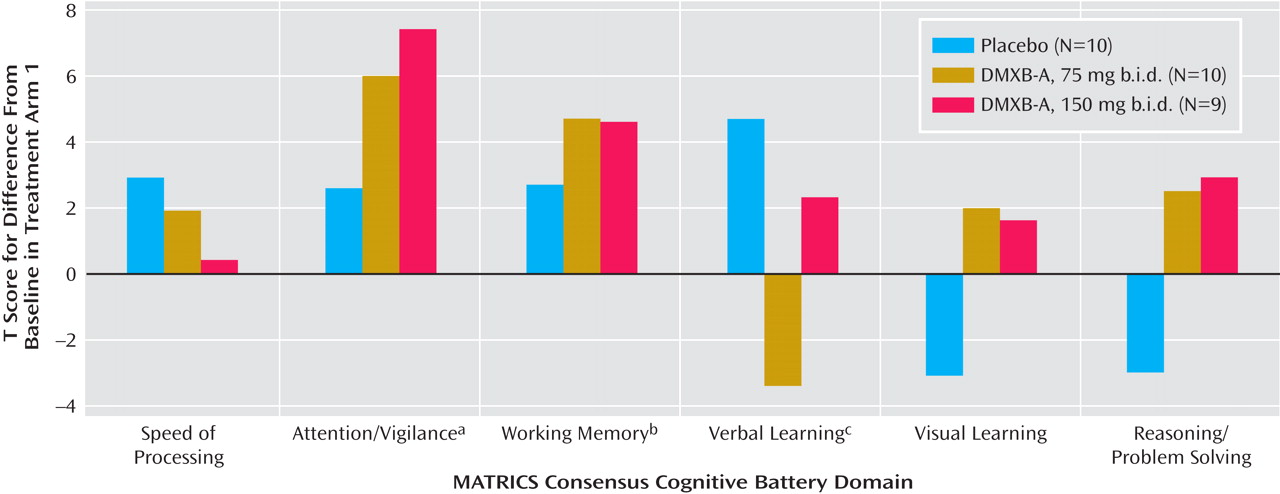
Therefore, we performed a secondary analysis using only the results of the first arm of the study, to minimize the effects of repetition of the tests (
Figure 1 ). This analysis has limited power, because the subjects’ performance could not be compared with their performance in the other two conditions. Ten received placebo, 10 received 75 mg b.i.d. of DMXB-A, and nine received 150 mg b.i.d. of DMXB-A. The verbal learning domain T score significantly increased with placebo, compared to baseline, and nonsignificantly decreased with 75 mg b.i.d. of DMXB-A and increased with 150 mg b.i.d. of DMXB-A. Two domains significantly improved over baseline with DMXB-A treatment in the first arm. The attention/vigilance domain T score did not significantly change over baseline with placebo, but it significantly increased with DMXB-A at both 75 mg b.i.d. (mean=6.1, SD=9.4) and 150 mg b.i.d. (mean=7.6, SD=10.6). The working memory domain T score also did not significantly change over baseline with placebo, but it showed a significant increase with 75 mg b.i.d. of DMXB-A (mean=4.6, SD=6.5) and a nearly significant increase with 150 mg b.i.d. (mean=4.5, SD=7.3).
The digit span subtest also did not show significant effects of DMXB-A treatment. For this measure, the effect of encounter number was also significant (F=8.10, df=2, 24, p=0.002). The least squares mean difference in numbers recalled between week 6 and week 16 was also significant, with a mean increase of 1.9 digits (SD=1.9) (t=3.30, df=27, p=0.003). In the first arm, there was improvement during both DMXB-A treatments, but not placebo, compared to baseline, but these effects were not significant.
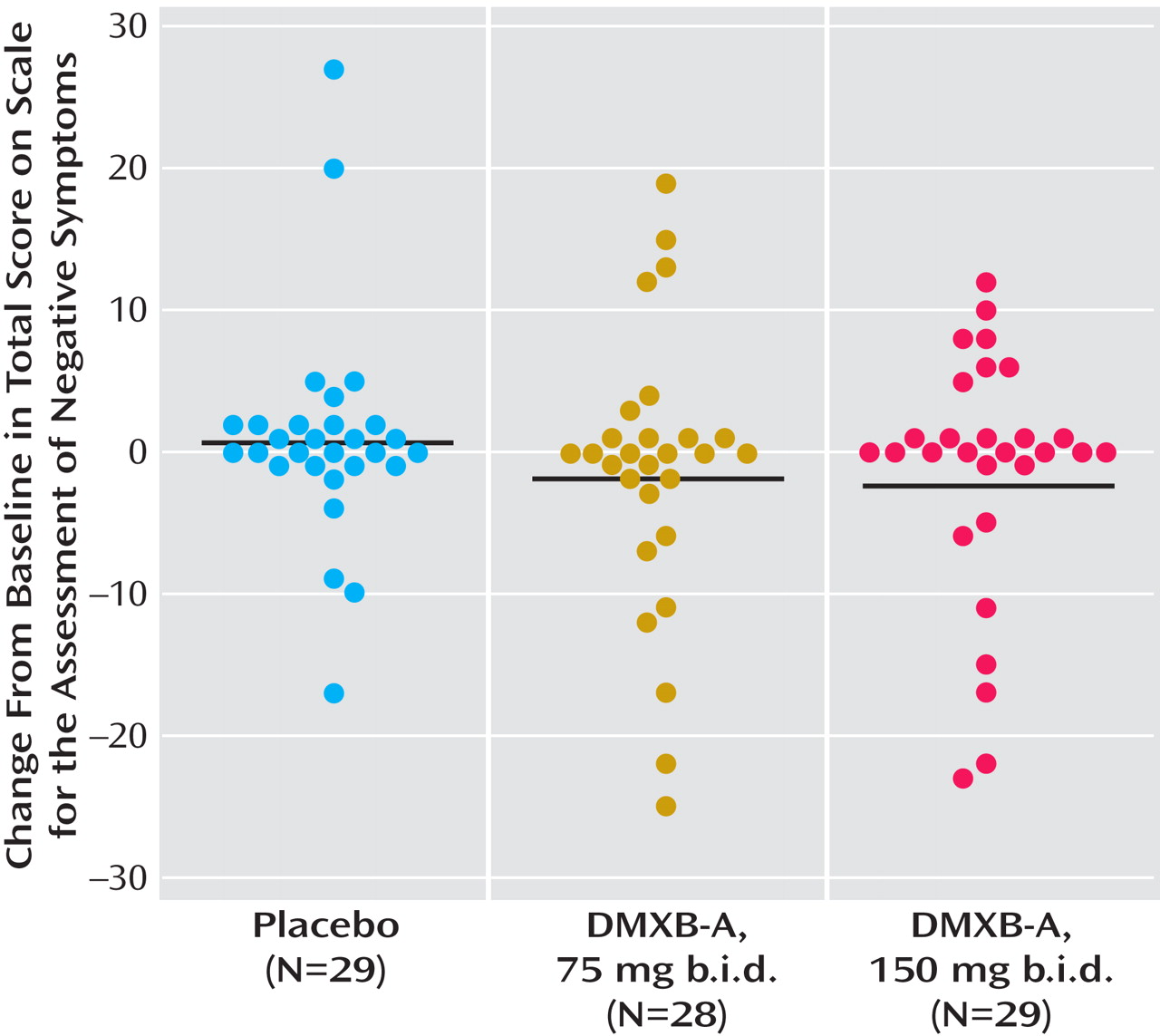
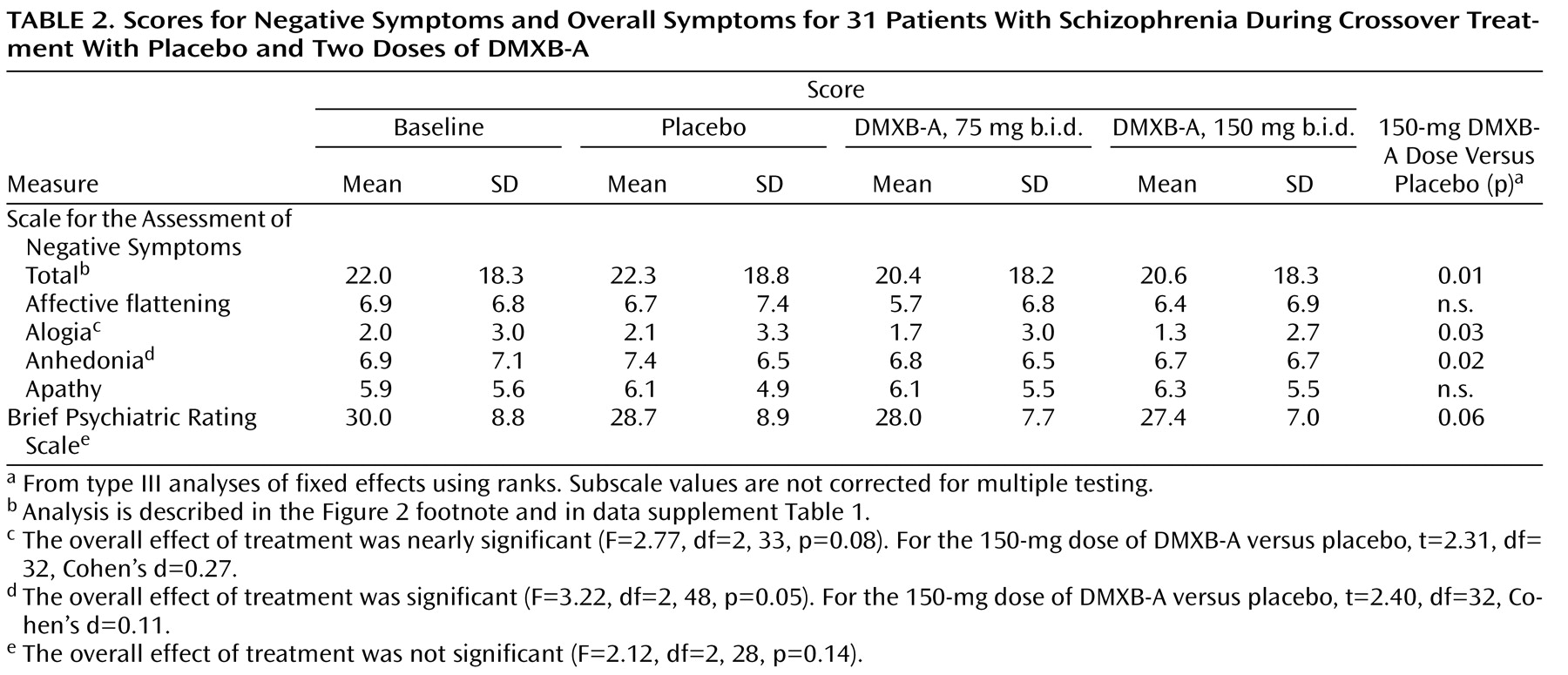
Significant effects of DMXB-A treatment were observed for the SANS total score (Figure 2, data supplement
Table 1 ). For 150 mg b.i.d. of DMXB-A, the mean improvement in ratings compared to placebo was 1.35 (SD=2.80). For 75 mg b.i.d. of DMXB-A, the improvement in ratings compared to placebo fell short of significance (mean=0.96, SD=2.85) (
Figure 2 ). Two of the subscales, alogia and anhedonia, showed significant effects of 150 mg b.i.d. of DMXB-A, compared to placebo (
Table 2 ).
The BPRS total score showed a nonsignificant effect of DMXB-A treatment with the same analysis used for the SANS. Only the contrast between 150 mg b.i.d. of DMXB-A and placebo approached significance in the nonparametric analysis (
Table 2 ).
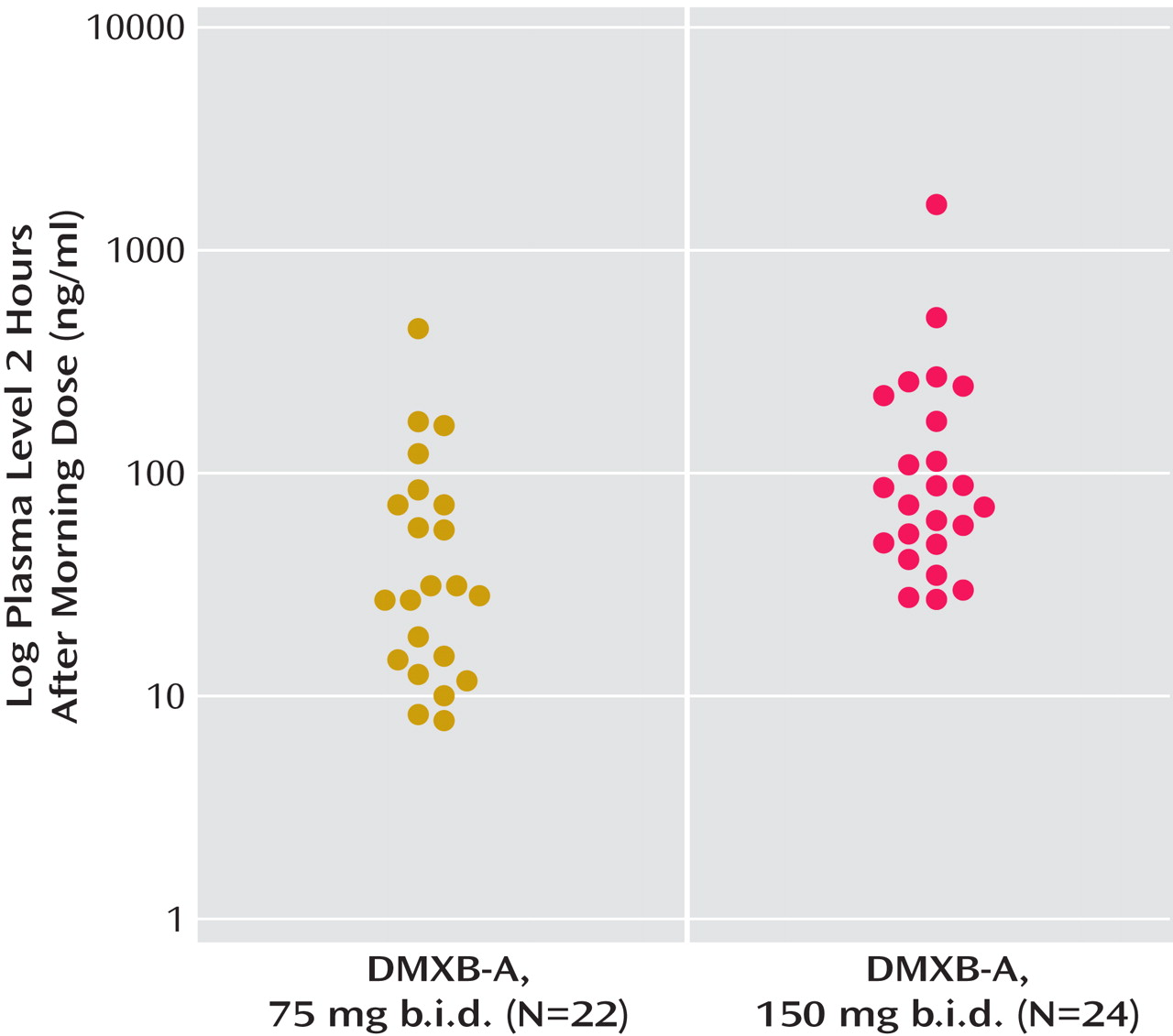
The mean plasma level of DMXB-A obtained after the morning dose on the last day of each 4-week treatment arm with DMXB-A was 50.7 ng/ml (SD=88.7) at the 75-mg dose and 129.9 ng/ml (SD=288.0) at the 150-mg dose. Plasma levels did not influence cognitive effects, symptoms, or any safety measure. One subject’s level was 445 ng/ml at the 75-mg dose and 1570 ng/ml at the 150-mg dose; both values are more than three standard deviations above the mean levels. This subject’s clinical response and serum chemistry results did not differ from the other subjects’ responses. The mean plasma levels were higher than the values previously found in the phase 1 study, 13.2 (SD=14.4) for the 75-mg dose and 23.2 (SD=16.0) for the 150-mg dose (
Figure 3 ).
Type of antipsychotic treatment—no treatment, first-generation antipsychotic, clozapine, or other second-generation antipsychotic—had significant effects only on the speed of processing domain of the MATRICS battery and did not alter the effects of DMXB-A treatment on any measure. There were no effects of site on any of the measures.
Adverse Effects
There was no statistically significant increase in adverse side effects reported by the subjects during DMXB-A treatment, compared with placebo (data supplement Table 2). There were increased reports of nausea and restlessness during DMXB-A treatment, but decreased reports of nervousness. Nausea occurred in 14 patients at the higher dose and is consistent with the known effects of nicotinic agonists on gastrointestinal mobility. None of these effects was severe. One subject became suicidal after 3 days of drug treatment (DMXB-A, 150 mg b.i.d.). He presented himself to an emergency room and was admitted to the hospital, because of a previous severe attempt. He made no attempt during this episode. He said that he had felt well during the treatment, but he became suicidal when his girlfriend told him that she was leaving him. He was removed from the study, but his suicidal ideation was not judged to be related to the study medication.
There were no significant effects of drug treatment on vital signs, ECG, or the results of urinalysis, hematology measurements, or serum chemistry tests (data supplement Table 3). Transient elevations of liver enzymes were observed in different subjects during all three treatment conditions. They had all resolved by the time of repeat testing and did not appear to be related to drug treatment.
Ratings on the Simpson-Angus Scale increased nonsignificantly with the increases in DMXB-A dose, from a mean score of 2.4 (SD=3.2) with placebo to 2.5 (SD=3.3) with 75 mg b.i.d. and 2.8 (SD=3.1) with 150 mg b.i.d. of DMXB-A. Increases were most apparent in head rotation; four of five subjects with ratings of minimal impairment during placebo treatment had increases in ratings to mild or moderate with one or both DMXB-A doses. Two subjects had minimal impairment with DMXB-A that was not observed with placebo. Five subjects had minimal to mild tremors during DMXB-A treatment that did not occur with placebo. Three subjects who had tremors with placebo had increased ratings during DMXB-A treatment, while two had decreases in ratings.
Discussion
The trial did not show significant effects of DMXB-A on cognition over the three treatment arms. What made detection of DMXB-A’s effect difficult may have been the strong effects of test repetition. Subjects improved markedly in their performance over 4 months. We had instituted a baseline test with the MATRICS Consensus Cognitive Battery and digit span subtest, intending to have most of the practice effects occur prior to the three treatment arms, but effects of practice continued throughout the trial. Because the MATRICS tests were chosen for their repeatability, this problem had not been anticipated, but use in a three-arm crossover design was not envisioned in the design of the MATRICS battery
(1) . Nevertheless, the practice effect would not have obscured a more robust drug effect.
The issue of practice effects has been raised as a possible explanation for the improved performance of patients with first-episode schizophrenia during treatment with second-generation antipsychotic drugs
(30) . Although none of our patients was in the first episode, a similar phenomenon appeared to occur in this study. We therefore examined performance during the first arm of the protocol only. The effects of DMXB-A after 4 weeks’ treatment on the T score for the attention/vigilance domain, which reflects performance on the Continuous Performance Test—Identical Pairs version, is noteworthy because performance on this test did not change after 6 weeks of treatment with second-generation antipsychotic drugs in first-episode patients
(30) . Significant effects on the T score for the working memory domain were also observed in the first arm. The decreased performance on the verbal learning domain T score observed in the first arm with 75 mg b.i.d. of DMXB-A reflects significant improvement in the number of items recalled during placebo and a nonsignificant decrease during DMXB-A treatment. The Hopkins Verbal Learning Test, which is used for this domain, asks subjects to learn a list of words. Subjects are given three trials to listen to and then repeat the list; the T score is derived from the sum of items recalled correctly during all three trials. A difference between 75-mg DMXB-A and placebo was observed only in the first learning trial. The test also measures delayed memory, retention, and later recognition of the words. Performance on these measures did not differ between treatments. Thus, viewing the test as a whole, there does not seem to be a strong negative effect of DMXB-A on verbal memory.
The SANS showed no effect of encounter number and did show a significant effect of DMXB-A treatment. The effect on core negative symptoms is also noteworthy, as these symptoms are generally resistant to antipsychotic drugs. Because this study was an initial phase 2 test, we did not establish a priori criteria for clinically significant effects. Many patients expressed that they were substantially more organized in their thoughts and actions, and several spontaneously reported their accomplishment of tasks at home that they had not been previously able to do.
The α
7 -nicotinic receptors activated by DMXB-A are both presynaptic and postsynaptic. The postsynaptic α
7 receptors are predominantly expressed on inhibitory interneurons, particularly in the hippocampus and nucleus reticularis thalami, where they inhibit thalamic input to the cerebral cortex. Activation of these receptors increases inhibitory neuron activity
(31) . In the phase 1 test, DMXB-A increased inhibition of P50 auditory evoked responses, and this effect is consistent with increased neuronal inhibition
(20) . It is possible that increased inhibition is the mechanism of improved attention/vigilance and working memory in this study, because inhibition of extraneous activity is necessary for these functions. Improved neurocognition would enable subjects to have better-functioning thought processes, which would be recognized as decreased alogia in the SANS. DMXB-A also increases the release of dopamine through activation of presynaptic receptors, which may account for the decrease in anhedonia observed with the drug
(32) .
There are other compounds currently in clinical use that have direct or indirect effects on α
7 -nicotinic receptors. Galantamine, an acetylcholinesterase inhibitor that is also an allosteric modulator of several nicotinic receptors, including the α
7 -nicotinic receptor, improved several aspects of cognition in schizophrenia and also improved the SANS alogia score
(33) . In contrast, rivastigmine, which does not have these allosteric properties, had no effect in schizophrenia
(34) . The most important drug with indirect effects on α
7 -nicotinic receptors is clozapine. Patients who respond well to clozapine normalize P50 inhibition and decrease their smoking
(35 –
37) . Animal model experiments show that clozapine’s neurobiological effects include activation of α
7 -nicotinic receptors, presumably through the increased release of acetylcholine in the hippocampus
(38) . The inclusion in this study of two patients taking clozapine may have obscured some of the effects of DMXB-A. However, clozapine, compared to haloperidol, does not improve ratings of alogia
(39), which improved with DMXB-A.
Plasma levels of DMXB-A were more variable than observed in the previous phase 1 tests, where DMXB-A was given for 1–5 days
(19,
20) . While the present study was not designed to be a pharmacokinetic study, changes in metabolism are a possible explanation. The short half-life of DMXB-A could lead to variance in levels if the plasma were sampled at slightly different points in time relative to drug ingestion. Although levels were higher in some patients in this study than previously observed, the finding that levels were undetectable before the first morning dose suggests that there is no accumulation of drug over time due to altered metabolism. The overnight clearing of the drug makes tachyphylaxis from residual drug levels an unlikely explanation for the failure to observe cognitive improvement over all three arms, although longer-term effects mediated by cellular mechanisms cannot be excluded.