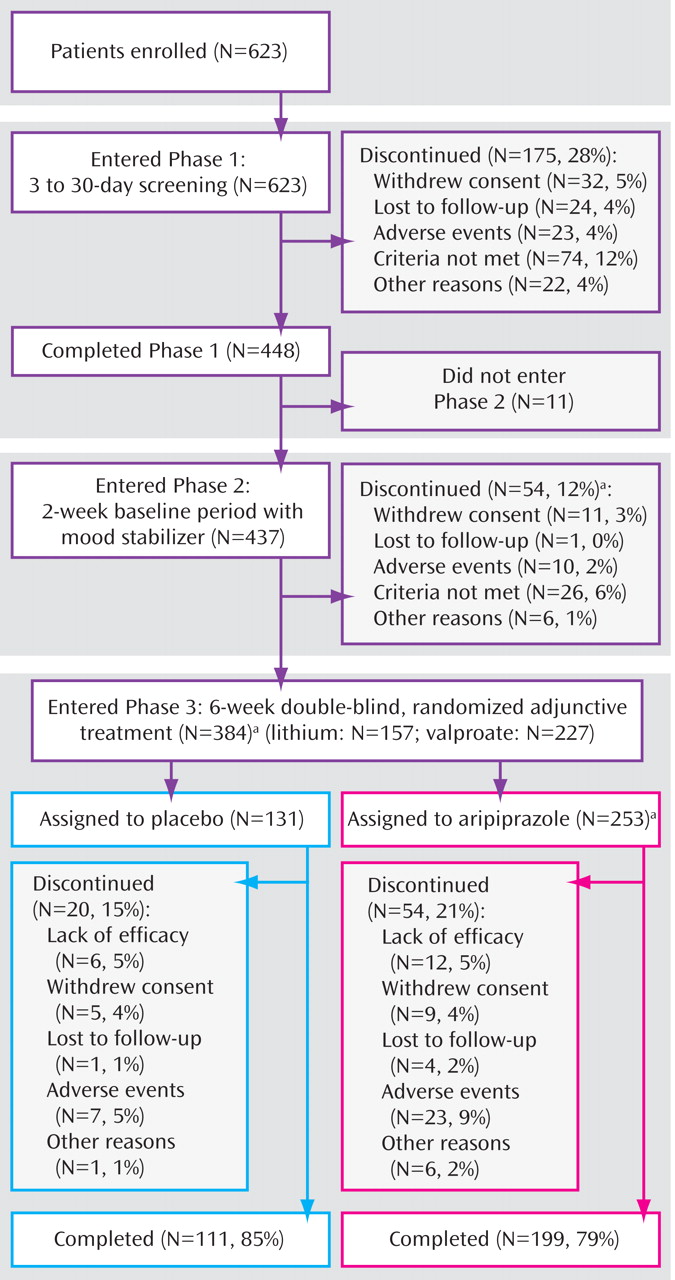
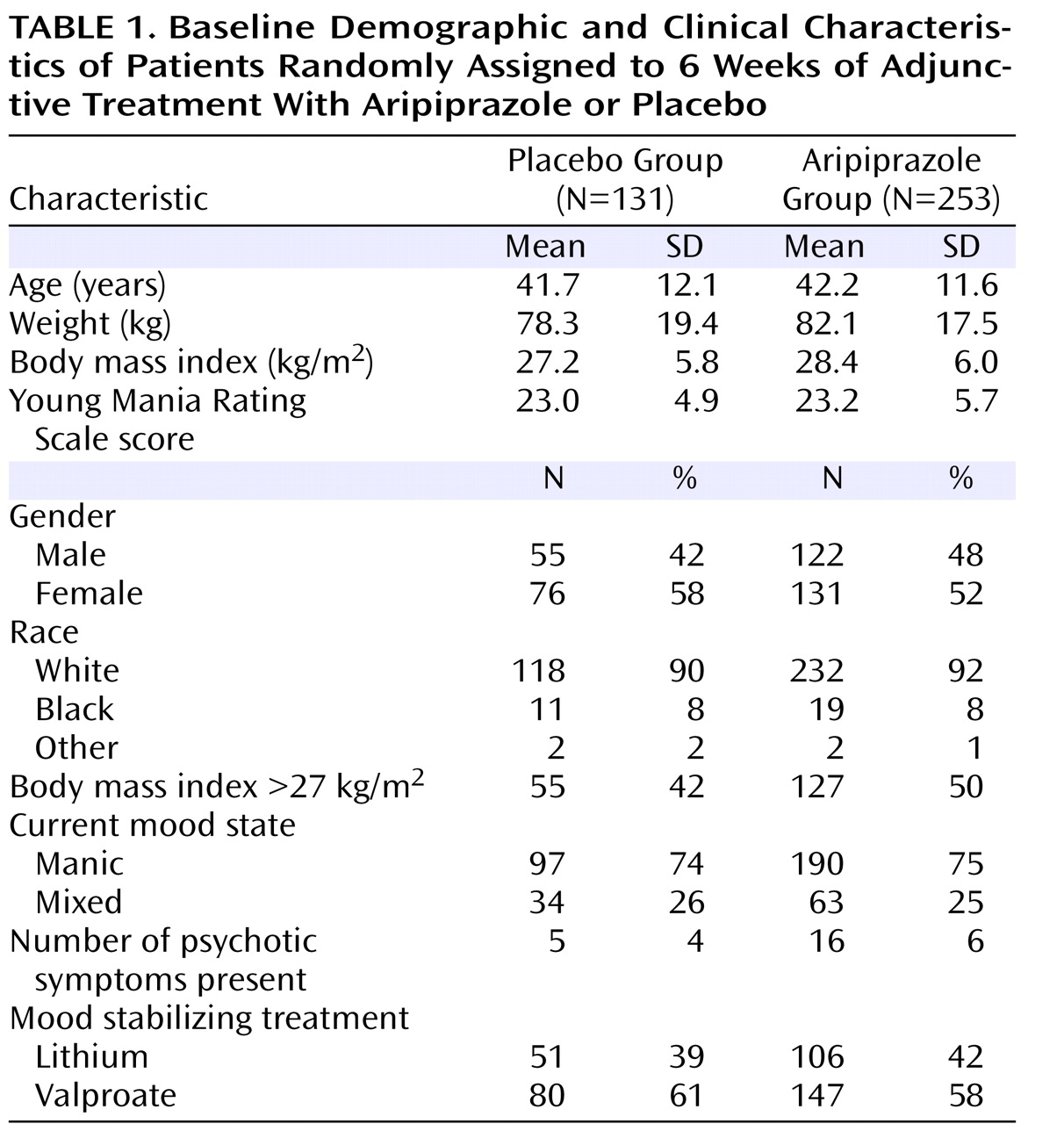
Study Treatments
Of the patients randomly assigned to double-blind treatment (N=384), 157 were receiving lithium and 227 were receiving valproate. For the patients assigned to adjunctive aripiprazole, the mean dose of aripiprazole during week 6 was 19.0 mg/day. For the lithium subgroup, the mean dose of lithium the day before starting adjunctive treatment was 994 mg/day and 1,119 mg/day for the placebo and aripiprazole arms, respectively. Mean lithium serum levels at baseline in these two groups were 0.77 mmol/liter (SD=0.17) and 0.78 mmol/liter (SD=0.22), respectively. The mean dose of lithium during week 6 of double-blind treatment was 985 mg/day and 1,160 mg/day for the placebo and aripiprazole groups, respectively. Mean lithium serum levels at week 6 (last observation carried forward) were similar in both groups (placebo: 0.72 mmol/liter [SD=0.22]; aripiprazole: 0.76 mmol/liter [SD=0.35]; t= −0.87, df=140, p=0.385). A similar proportion of patients in both arms had a serum lithium level within the therapeutic range at endpoint (placebo: 68.0%; aripiprazole: 69.9%). For the valproate subgroup, the mean dose the day before starting adjunctive treatment was 1,175 mg/day and 1,180 mg/day for the placebo and aripiprazole arms, respectively. The mean serum valproate level at baseline in the two groups was 77.2 μg/ml (SD=23.4) and 77.8 μg/ml (SD=21.0), respectively. The mean dose of valproate during week 6 of double-blind treatment was 1,179 mg/day and 1,225 mg/day for the placebo and aripiprazole groups, respectively. Similar mean serum valproate levels at endpoint (week 6; last observation carried forward) were seen between the two groups (placebo: 68.35 μg/ml [SD=23.92]; aripiprazole: 68.23 μg/ml [SD=23.63]; t=0.04, df=161, p=0.971). A similar proportion of patients in both arms had a serum valproate level within the therapeutic range at endpoint (placebo: 78.8%; aripiprazole: 80.0%).
More than 40% of patients received concomitant CNS medication. The most frequently taken CNS medications were anxiolytics (placebo: 24.6%; aripiprazole: 20.9%) and other analgesics and antipyretics (placebo: 23.1%; aripiprazole: 21.3%). A total of 47 (18.6%) patients in the aripiprazole group received a concomitant medication for the potential treatment of extrapyramidal symptoms, compared with nine (6.9%) patients in the placebo group.
Efficacy
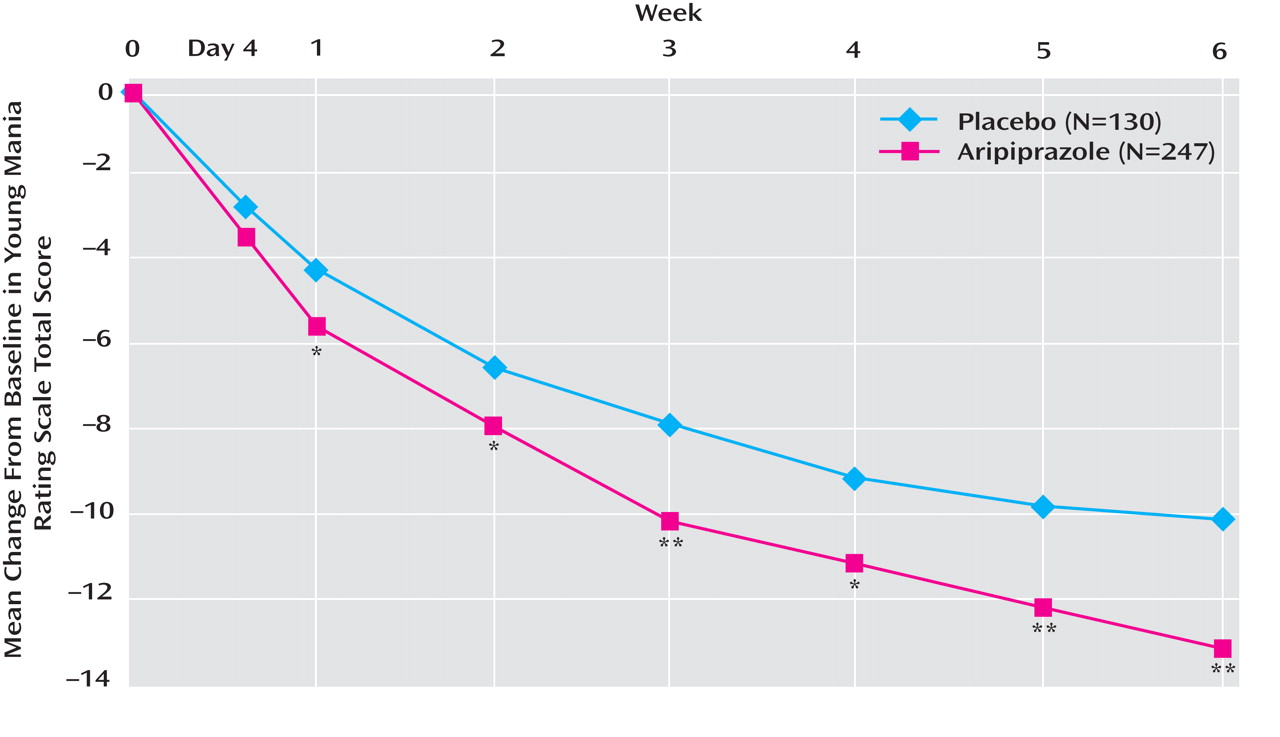
At week 6, adjunctive aripiprazole showed significantly greater improvements from baseline in Young Mania Rating Scale total score than placebo (–13.3 [SD=7.9] versus –10.7 [SD=7.6]; F=9.54, df=1, 373, p<0.01; d=0.33) (
Figure 2 ). Aripiprazole plus mood stabilizer produced significantly greater improvements from baseline in Young Mania Rating Scale total score than lithium/valproate monotherapy by week 1 and at all subsequent endpoints (p<0.05) (
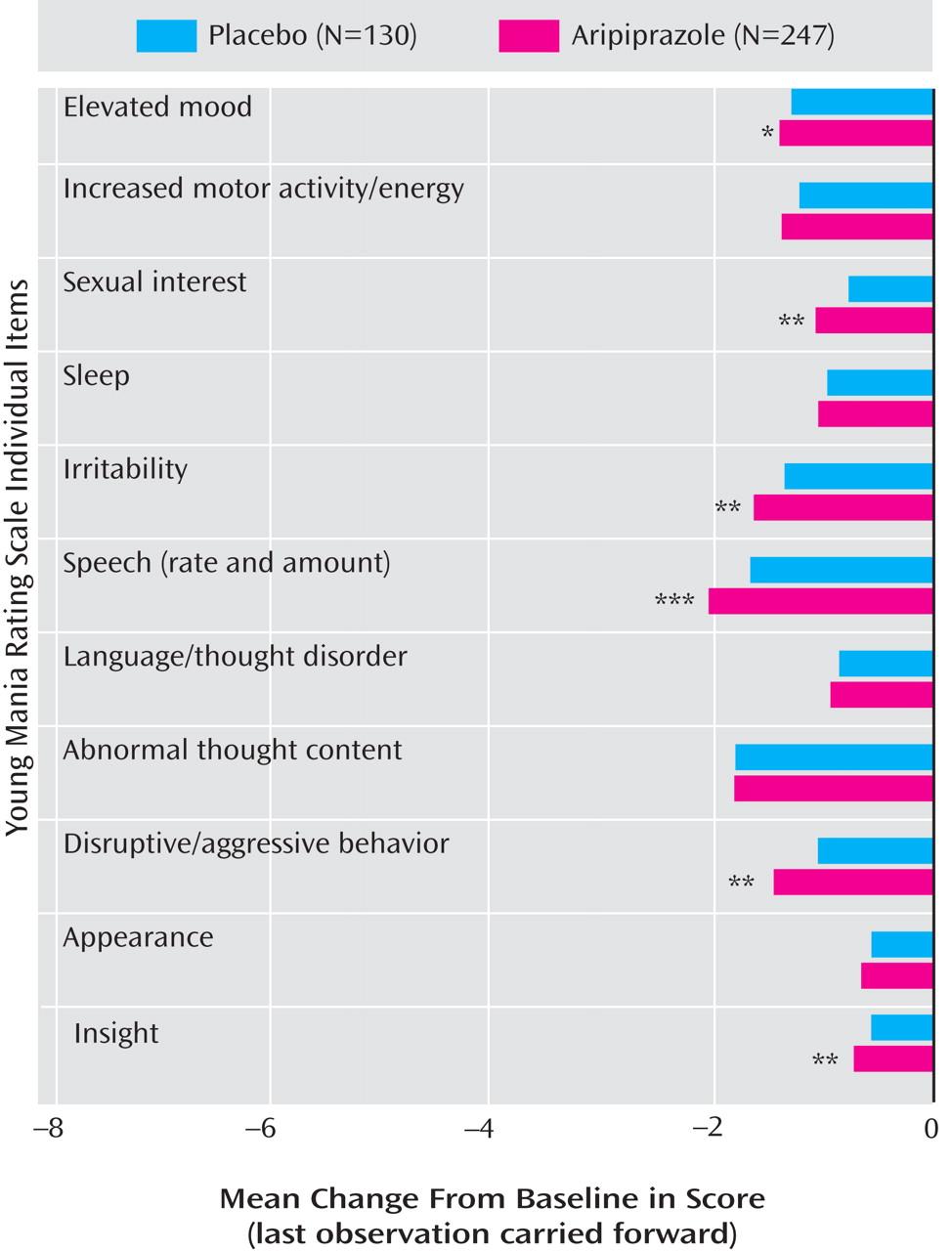
Figure 2 ). In a post-hoc analysis, adjunctive aripiprazole did not worsen manic symptoms as measured by an item analysis of the Young Mania Rating Scale, and in addition demonstrated significant improvements relative to placebo on six of the 11 items: elevated mood, sexual interest, irritability, speech, disruptive/aggressive behavior, and insight (
Figure 3 ).
A priori analyses of each subgroup showed that adjunctive aripiprazole with valproate produced significantly (p<0.05) greater improvements in Young Mania Rating Scale total score than placebo from day 4 through week 6 (–14.0 [SD=7.8] versus –10.7 [SD=7.9]; F=9.06, df=1, 222, p<0.01; last observation carried forward). For the lithium subgroup, the improvement from baseline at week 6 with aripiprazole was numerically, albeit not significantly, greater than with adjunctive placebo (–12.4 [SD=8.1] versus –10.8 [SD=7.1]; F=1.40, df=1, 149, p=0.238; last observation carried forward). In the observed data, adjunctive aripiprazole produced significantly greater improvements in Young Mania Rating Scale total score than adjunctive placebo at week 5 (–13.7 [SD=6.8] versus –10.2 [SD=7.5]; F=6.24, df=1, 114, p<0.05) and week 6 (–14.7 [SD=6.5] versus –10.2 [SD=7.4]; F=11.00, df=1, 113, p≤0.001).
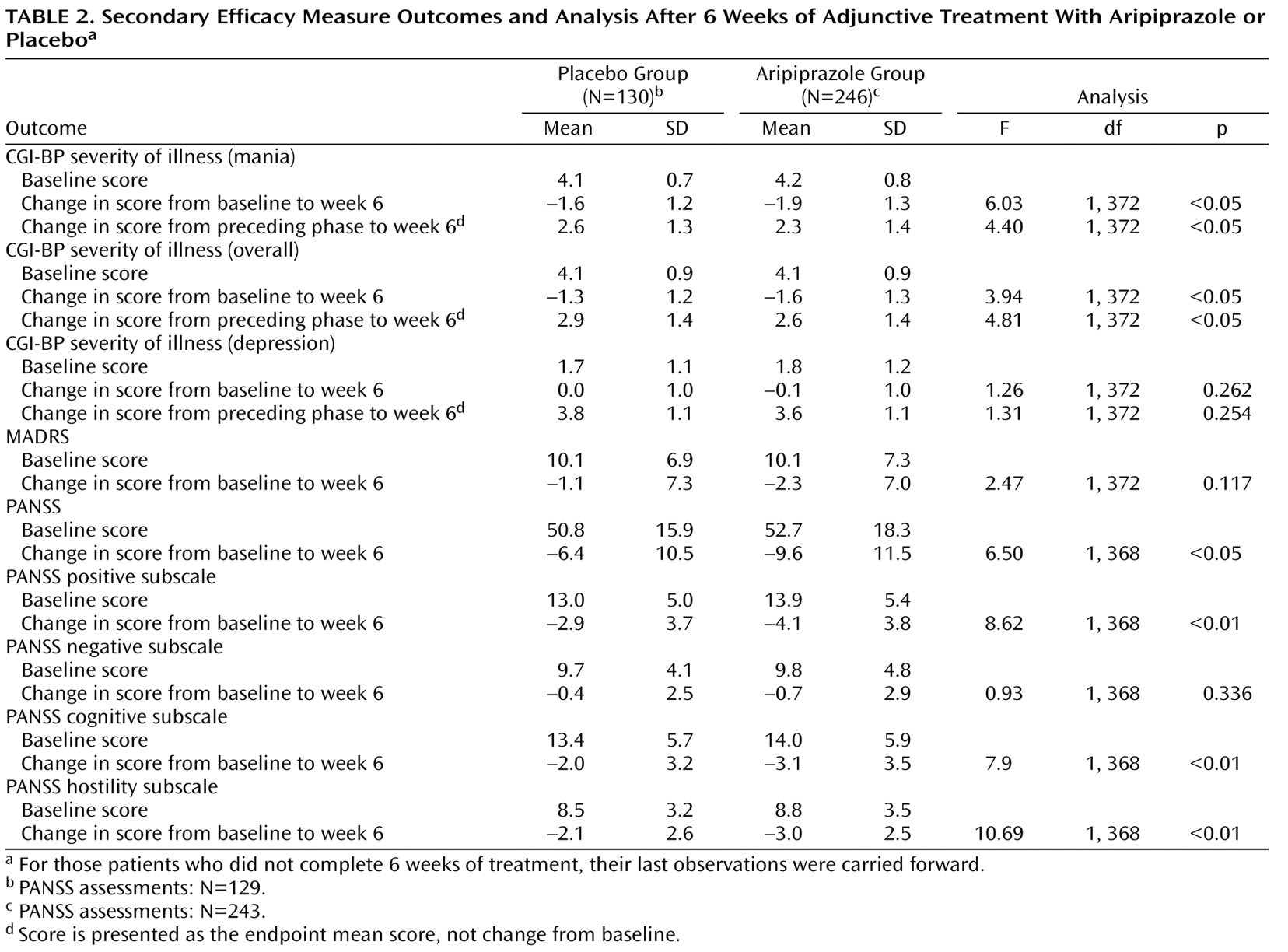
Adjunctive aripiprazole was also associated with significant reductions in CGI-BP severity of illness (mania) score versus placebo (
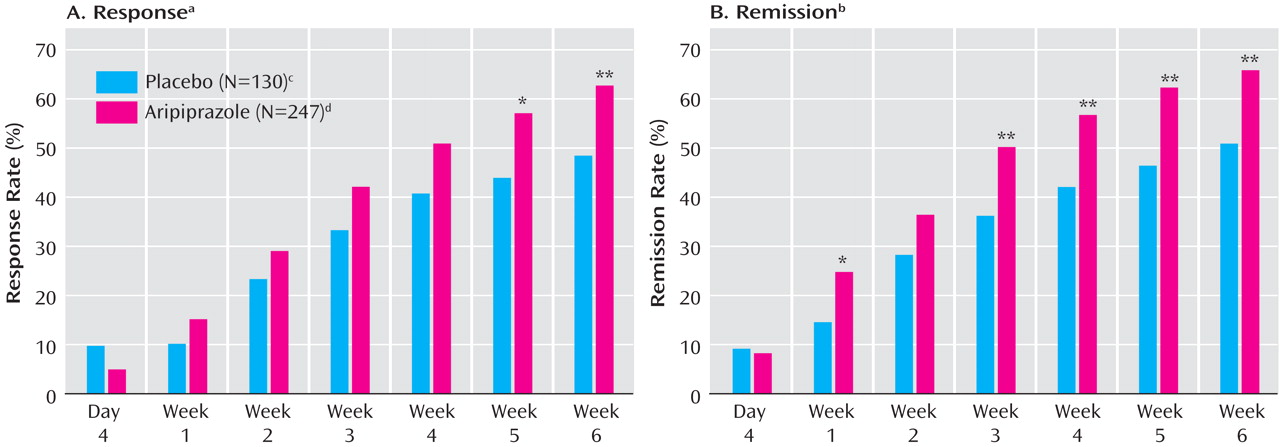
Table 2 ). Response rates were significantly higher with adjunctive aripiprazole than with placebo at week 5 (χ
2 =5.79, df=1, p≤0.05; last observation carried forward) (
Figure 4 ). At week 6, 62.8% of the aripiprazole group had responded to treatment, compared with 48.5% of the placebo group (χ
2 =7.07, df=1, p<0.01; last observation carried forward), and the number needed to treat was 7. Compared with placebo, aripiprazole also produced significantly higher remission rates at weeks 1, 3, 4, 5, and 6 (
Figure 4 ). At week 6, the remission rate was 66.0% for aripiprazole and 50.8% for placebo (χ
2 =8.18, df=1, p<0.01; last observation carried forward), and the number needed to treat was 7. Post-hoc analysis using more stringent remission criteria (Young Mania Rating Scale total score ≤12 plus a MADRS total score ≤8) also showed significantly higher remission rates with aripiprazole than with placebo at week 6 (50.2% versus 36.4%; χ
2 =6.44, df=1, p<0.05; last observation carried forward). Similar rates were observed in the lithium and valproate subgroups, although the differences between the two were not statistically significant (lithium: 51.0% versus 34.7%; χ
2 =3.51, df=1, p=0.061; valproate: 49.7% versus 37.5%; χ
2 =3.06, df=1, p=0.080).
Time to response (number of events for aripiprazole: 178 [72.1%]; for placebo: 83 [63.8%]; hazard ratio=1.45) and time to remission (number of events for aripiprazole: 185 [74.9%]; for placebo: 87 [66.9%]; hazard ratio=1.48), as evaluated by survival curves, were both significantly in favor of aripiprazole (χ 2 =7.84, df=1, p<0.01 and χ 2 =9.04, df=1, p<0.01, respectively).
Adjunctive aripiprazole also produced a significantly greater improvement than placebo in the CGI-BP severity of illness (overall) score at week 6 (p<0.05; last observation carried forward) and significantly greater change than placebo from preceding phase in CGI-BP severity of illness (overall) and (mania) scores (both p values <0.05; last observation carried forward) (
Table 2 ). The improvement over placebo in MADRS score seen with aripiprazole was not statistically significant at endpoint (last observation carried forward). Analysis of the observed data demonstrated statistically significant improvements in MADRS scores for adjunctive aripiprazole versus adjunctive placebo at week 1 (–2.0 [SD=4.2] versus –0.2 [SD=5.8]; F=10.79, df=1, 343, p<0.01), week 5 (–3.8 [SD=4.9] versus –2.6 [SD=5.6]; F=4.03, df=1, 303, p<0.05), and week 6 (–3.5 [SD=6.2] versus –1.7 [SD=6.6]; F=5.90, df=1, 302, p<0.05).
The proportion of patients with emergent depression was significantly lower in the aripiprazole arm than the placebo arm (7.7% versus 16.9%; χ 2 =7.61, df=1, p<0.01). Similarly, fewer patients reported emergent depression in the aripiprazole arm for both the lithium subgroup (7.8% versus 24.0%; χ 2 =7.61, df=1, p<0.01) and valproate subgroup, although the difference in the valproate subgroup was not statistically significant (7.6% versus 12.5%; χ 2 =1.42, df=1, p=0.233).
Significantly greater improvements from baseline to week 6 were evident for aripiprazole compared with placebo in PANSS total score and PANSS positive, cognitive, and hostility subscale scores (p≤0.05; last observation carried forward), although there was no significant difference between treatments in PANSS negative subscale scores (
Table 2 ). There was a significant difference between groups in mean change from baseline to week 6 in Longitudinal Interval Follow-Up Evaluation—Range of Impaired Function Tool total score favoring aripiprazole versus placebo (–1.8 versus –1.0; p=0.046).
Safety
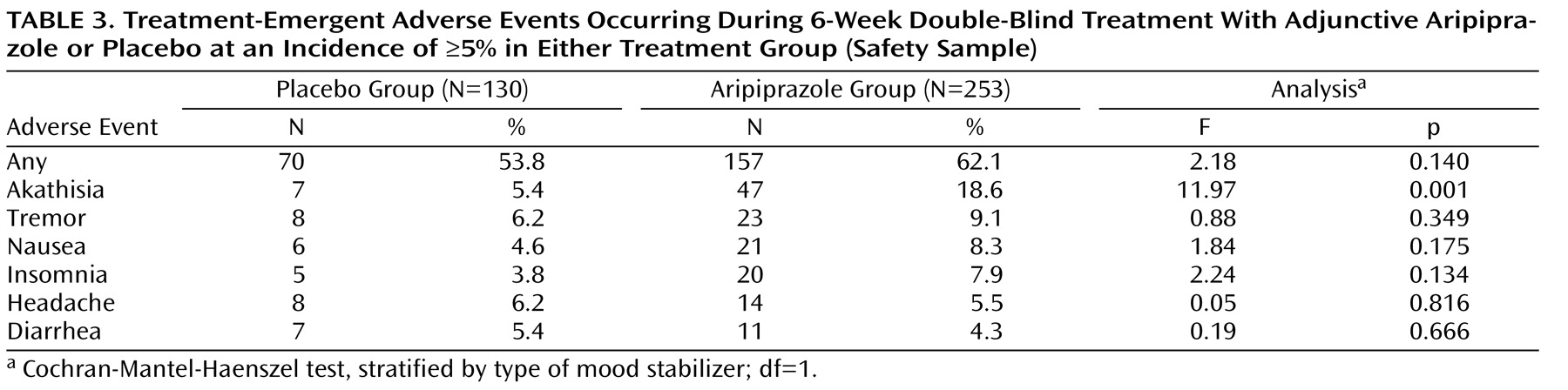
During double-blind treatment, 53.8% (N=70) of patients in the adjunctive placebo group and 62.1% (N=157) of patients in the adjunctive aripiprazole group reported at least one treatment-emergent adverse event (p=0.140). Adverse events occurring at an incidence ≥5% in either treatment group are shown in
Table 3 .
During double-blind treatment, serious adverse events were reported for 3.2% of aripiprazole patients and 2.3% of placebo patients; most frequent were psychiatric disorders typical of the patient population (aripiprazole: 2.4%; placebo: 2.3%). Most serious adverse events were judged to be unrelated or unlikely to be related to study medication.
Patients treated with adjunctive aripiprazole showed more extrapyramidal symptoms than patients in the placebo group. Adverse events related to treatment-emergent extrapyramidal symptoms were reported in 28.1% (N=71) of the aripiprazole group and 13.8% (N=18) of the placebo group. Of these patients, 25.4% (N=18) of the aripiprazole group and 22.2% (N=4) of the placebo group had resolved the adverse event by study end. The most frequently reported extrapyramidal symptom-related adverse event was akathisia (aripiprazole: 18.6%; placebo: 5.4%). Other extrapyramidal symptom-related adverse events occurred in <10% of patients in either group: tremor (placebo: 6.2%; aripiprazole: 9.1%); extrapyramidal disorder (placebo: 0.8%; aripiprazole: 4.7%); hypertonia (placebo: 0%; aripiprazole: 0.4%); hypokinesia (placebo: 0%; aripiprazole: 0.4%); muscle spasms (placebo: 0.8%; aripiprazole: 2.0%); dyskinesia (placebo: 0.8%; aripiprazole: 0.4%); and muscle twitching (placebo: 0%; aripiprazole: 0.4%). The lithium subgroup showed higher rates of akathisia (aripiprazole: 28.3%; placebo: 4.0%) and tremor (aripiprazole: 13.2%; placebo: 8.0%) than the valproate subgroup (aripiprazole: 11.6%; placebo: 6.3% and aripiprazole: 6.1%; placebo: 5.0%, respectively). Akathisia was generally mild to moderate in severity (aripiprazole: 15.8%; placebo: 4.6%); severe symptoms were reported in 2.8% and 0.8% of aripiprazole and placebo patients, respectively. For aripiprazole patients who reported akathisia with a maximum severity of mild, moderate, and severe, the corresponding median highest Barnes Rating Scale for Drug-Induced Akathisia score was 2, 3, and 4, respectively. The majority of akathisia in the aripiprazole group began in the first 3 weeks of treatment (89.4%). Akathisia occurring during double-blind treatment led to discontinuation in 13 aripiprazole patients (5.1%) and one placebo patient (0.8%). By the end of double-blind treatment, akathisia had resolved in 15 out of the 47 patients (31.9%) in the aripiprazole group and one out of the seven patients (14.3%) in the placebo group reporting this symptom. The median duration of resolved akathisia events was 8 days. For those subjects in the aripiprazole arm whose akathisia resolved during the study, two had their study medication dose adjusted (one patient received a dose reduction and one patient discontinued the study). Permitted concomitant medications (e.g., benztropine, propranolol) for akathisia were administered in 11 patients (73.3%). Akathisia resolved without intervention in three patients. The one patient in the placebo arm whose akathisia resolved at the end of the double-blind treatment phase had a dose reduction but did not receive any concomitant medication to treat the akathisia.
During adjunctive treatment, significant mean changes from baseline to the end of week 6 (last observation carried forward) were seen in Simpson-Angus Scale total scores (placebo: baseline score=10.5, change in score=0.07; aripiprazole: baseline score=10.5, change in score=0.73; F=9.05, df=1, 370, p<0.01) and Barnes Rating Scale for Drug-Induced Akathisia scores (placebo: baseline score=0.18, change in score=0.11; aripiprazole: baseline score=0.15, change in score=0.30; F=5.03, df=1, 370, p=0.026). Mean changes in AIMS total scores were not significantly different between treatment groups (placebo: baseline score=0.18, change in score= –0.10; aripiprazole: baseline score=0.25, change in score=0.08; F=3.57, df=1, 370, p=0.060).
Small changes from baseline in body weight were observed with aripiprazole (+0.55 kg [SD=2.66]) and placebo (+0.23 kg [SD=3.51]) at endpoint (last observation carried forward), with no statistically significant differences between groups (p=0.331). There were similar findings in the observed data (aripiprazole: +0.67 kg [SD=2.77]; placebo: +0.41 kg [SD=3.64]; p=0.499). There were no significant treatment differences in the mean change in weight from baseline to week 6 (last observation carried forward) in the lithium or valproate subgroups. The percentage of patients showing clinically significant weight gain (≥7% increase in weight from baseline to week 6; last observation carried forward) was similar in the two groups: 3.9% (N=5) in the placebo arm and 3% (N=7) in the aripiprazole arm. There were similar findings in the observed data and in the lithium and valproate subgroups.
There were no clinically meaningful differences between treatments in laboratory parameters, including total cholesterol, high- and low-density lipoprotein cholesterol, glucose and triglycerides, and serum prolactin levels. Adjunctive aripiprazole plus valproate/lithium treatment was not associated with any clinically relevant changes in vital sign measurements or any ECG abnormalities.