The anatomical brain changes in first-episode schizophrenia are of interest for at least three reasons: 1) they may indicate a progression of brain changes after disease onset, 2) they may represent the core regions of pathological change in schizophrenia, and 3) they may provide a key to earlier diagnosis.
Changes in brain structure in first-episode schizophrenia have been identified by meta-analyses of MRI studies
(1 –
3) . Compared with matched comparison groups, patients were found to have reduced whole brain volume (97%) and increased lateral ventricular volume (134% on the right and 125% on the left). In patients, hippocampal volumes were 92% on both sides
(1) . However, amygdala volumes and temporal lobe volumes were not significantly different
(3) .
These changes may be less widespread than those in chronic schizophrenia. Meta-analyses that included mainly patients with chronic schizophrenia
(4 –
6) found that patients also had smaller mean cerebral volumes (98%) and greater total ventricular volumes (126%). Other volumes that were smaller were the hippocampus (93% on the left and 94% on the right), the parahippocampi (92% on the left and 89% on the right), the amygdala (91% on both sides), the frontal lobes (97% on both sides), and the temporal lobes (98% on the left and 97% on the right)
(5) .
If there are more widespread brain changes in chronic schizophrenia than in first-episode schizophrenia, then this could imply a progression of brain anatomical changes after symptom onset. Since hippocampal deficits, but not amygdala deficits, appear to be present in first-episode schizophrenia, this has led to the hypothesis that there is a progression of temporolimbic involvement after disease onset
(3) . Furthermore, if there are more localized changes in first-episode schizophrenia, then these may represent the core regions of pathology (or neurodevelopmental abnormality). They may represent the critical nodes in neurochemical circuits whose continuing dysfunction leads to further anatomical changes within the circuits over time.
Finally, the identification of specific anatomical brain changes in first-episode schizophrenia could provide a key to earlier diagnosis. Studies of brain MR images in individuals at high risk of developing a psychotic disorder have found gray matter changes
(7,
8), and it is important to define which of these changes are indicative of first-episode schizophrenia. There is some evidence that earlier diagnosis and treatment of schizophrenia lead to improved outcomes
(9,
10) .
In this study, we conducted meta-analyses of voxel-based morphometry imaging studies by applying the technique of activation likelihood estimation
(11) . Activation likelihood estimation was originally developed to identify the brain regions that were consistently activated by a cognitive task
(12,
13), using coordinates reported by different functional imaging studies. It assumes that although each study reported the specific coordinates of activations, technical issues (such as interindividual variability in brain anatomy) and differences in investigators’ labels for anatomical regions lead to some uncertainty as to the actual locations of these peaks. Therefore, activation foci do not represent single points but rather “localization probability distributions” centered on the particular coordinates. In activation likelihood estimation, the foci reported by each study are modeled as a probability distribution. Then a map of the whole brain is constructed, assigning to each voxel a value equal to the probability that an activation lies within the voxel. This value is called the “activation likelihood estimation.” A statistical test of these values is then performed by comparing them with values in a null distribution obtained by permutation testing, correcting for multiple comparisons by controlling the false discovery rate. For example, a false discovery rate correction guarantees that in a set of voxels deemed significant for a test of α=0.05, the expected proportion of false positives is controlled
(11) .
One of the difficulties when comparing imaging studies is that there is considerable variability when labeling neuroanatomical regions, and differences in nomenclature could obscure findings. An advantage of the activation likelihood estimation technique is that because it uses the coordinates of reported foci (rather than anatomical labels) for meta-analysis, it avoids the problem of any mislabeling of regions in the primary literature
(14) .
In voxel-based morphometry, MR images are analyzed for structural change at the level of voxels—the individual elements within a three-dimensional digital image
(15,
16) . This automated technique analyzes the whole brain for changes rather than selecting a subsample of regions and so may be less likely to miss changes than the more traditional region-of-interest morphometry. We call this method
anatomical likelihood estimation (ALE) when applied to voxel-based structural imaging studies, since the primary studies measure brain changes in gray matter structure rather than brain activations (as in functional imaging studies).
A previous meta-analysis of gray matter changes in schizophrenia (including mainly studies of chronic schizophrenia) found that deficits in patients were more frequently observed in various cortical and subcortical regions, including the superior temporal gyrus bilaterally, the medial frontal gyrus bilaterally, the anterior cingulate, the insular cortex bilaterally, the parahippocampal gyrus bilaterally, the thalamus, and the caudate bilaterally
(17) .
The objectives of this meta-analysis were to use ALE to investigate gray matter structural brain changes in first-episode schizophrenia and to compare the distribution of these changes with those in chronic schizophrenia. We hypothesized that ALE analysis of first-episode schizophrenia studies would 1) identify the hippocampi but not the amygdala as regions affected by first-episode schizophrenia and 2) demonstrate more widespread cortical changes in chronic schizophrenia compared with first-episode schizophrenia.
Method
Study Ascertainment
Studies were considered for inclusion if they were published before June 2007 in article format (rather than as letters or abstracts), if they compared a group of subjects with schizophrenia (or schizophrenia and related diagnoses) and a comparison group (either related or unrelated to the subjects), if they utilized voxel-based morphometric analysis of MRI data sets to investigate differences in whole-brain structure (gray matter density), and if they reported the three-dimensional coordinates of brain changes in stereotactic space.
If measurements were reported for both an unrelated and a related comparison group, then the former measurements were used. If the comparison groups were unaffected dizygotic or monozygotic twins, then the former measurements were used (on the assumption that they showed genetic as well as environmental differences). Studies reporting results on a patient group in which patients had a diagnosis of schizophrenia but some patients had related diagnoses (e.g., schizoaffective disorder, first-episode psychosis) were also included. Studies that reported separate results for male and female subjects were entered as combined data. Studies in which the patients’ duration of illness was less than 1.5 years (e.g., with early-onset schizophrenia) were classified in the first-episode schizophrenia group.
Study data were excluded if insufficient data were reported to extract the number of subjects in each group, if there were fewer than six subjects in either the schizophrenia group or the comparison group, or if the data contributed to another publication, in which case the publication with the largest group size was selected.
A systematic search strategy was used to identify relevant studies. First, we carried out a MEDLINE search using the following keywords: schizophrenia, psychosis, first episode, MRI, voxel, SPM (statistical parametric mapping), Talairach; the search was conducted in June 2007, and no time span was specified for date of publication. Second, a manual search was conducted of the titles of published papers in three psychiatric journals for the period January 2007 to May 2007: the
American Journal of Psychiatry,
Archives of General Psychiatry, and
Biological Psychiatry . Finally, we searched the reference lists of the studies identified for inclusion. Studies were independently ascertained and checked by the authors.
Table 1 lists the articles included in the meta-analysis
(18 –
44) .
Coordinates that were reported in the stereotactic space of the Montreal Neurological Institute (MNI) were converted to Talairach coordinates using the Lancaster transform (icbm2tal) in GingerALE
(11) . Talairach coordinates that had been generated by the Brett transform applied to statistical parametric mapping MNI coordinates were transformed back to MNI space in GingerALE and then to Talairach space using the Lancaster transform. Coordinates produced using small-volume correction were included.
Of the structural neuroimaging studies considered for the meta-analyses, two studies
(45,
46) were excluded because the subjects overlapped with other included studies.
Statistical Analysis
Meta-analyses were performed when at least two studies providing coordinates suitable for meta-analysis were available. Meta-analyses were carried out using the activation likelihood estimation technique
(12) implemented in GingerALE (
11 ; www.brainmap.org/ale/).
Meta-analyses were performed using the Talairach stereotactic coordinates derived from the studies listed in
Table 1 . The likelihood of anatomical differences between groups was estimated on the basis of the equally weighted coordinates reported by the primary studies
(12) . The reported loci of maximal anatomical difference were modeled as the peaks of three-dimensional Gaussian probability density functions with full-width half-maximum of 10 mm. The probabilities were combined to form a map of the ALE score at each voxel. Using a permutation test (5,000 permutations), nonparametric estimates for p values were derived for ALE scores. These probability maps were thresholded, controlling the false discovery rate at p<0.05 and a cluster extent threshold of 100 voxels.
For each cluster, the coordinate of the weighted center was generated and the maximum ALE value within the cluster was identified. The Talairach location of the cluster was assigned by identifying the location of the coordinate of the maximum ALE value in the automated Talairach atlas (
47 ; http://www.talairach.org/), and this was manually checked in the Talairach atlas
(48) .
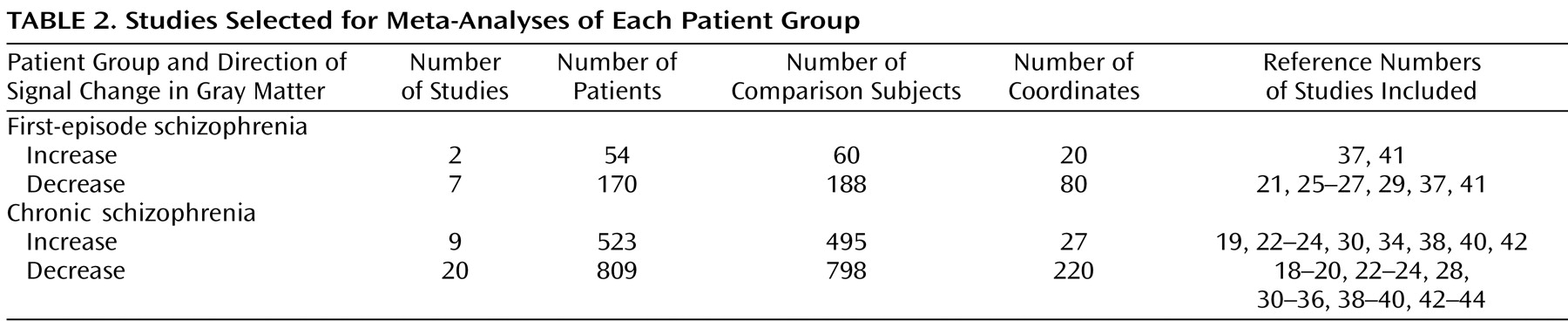
There were sufficient studies to perform meta-analyses (compared with comparison subjects) of decreases in gray matter in subjects with first-episode schizophrenia and in subjects with chronic schizophrenia, and increases in gray matter in subjects with first-episode schizophrenia and chronic schizophrenia.
In order to compare gray matter decreases in first-episode schizophrenia and chronic schizophrenia, a subtraction meta-analysis was performed
(11) . Subtraction meta-analysis yields an ALE map that shows regions in which the two groups of foci are significantly different. Since there were more studies and considerably more coordinates for gray matter decreases in chronic schizophrenia (N=220) than in first-episode schizophrenia (N=80), this could have resulted in the appearance of more extensive changes in chronic schizophrenia. Therefore, a meta-analysis was performed using a random sample of 80 coordinates from the chronic schizophrenia gray matter decreases in comparison with the 80 coordinates from the first-episode schizophrenia gray matter decreases.
Results
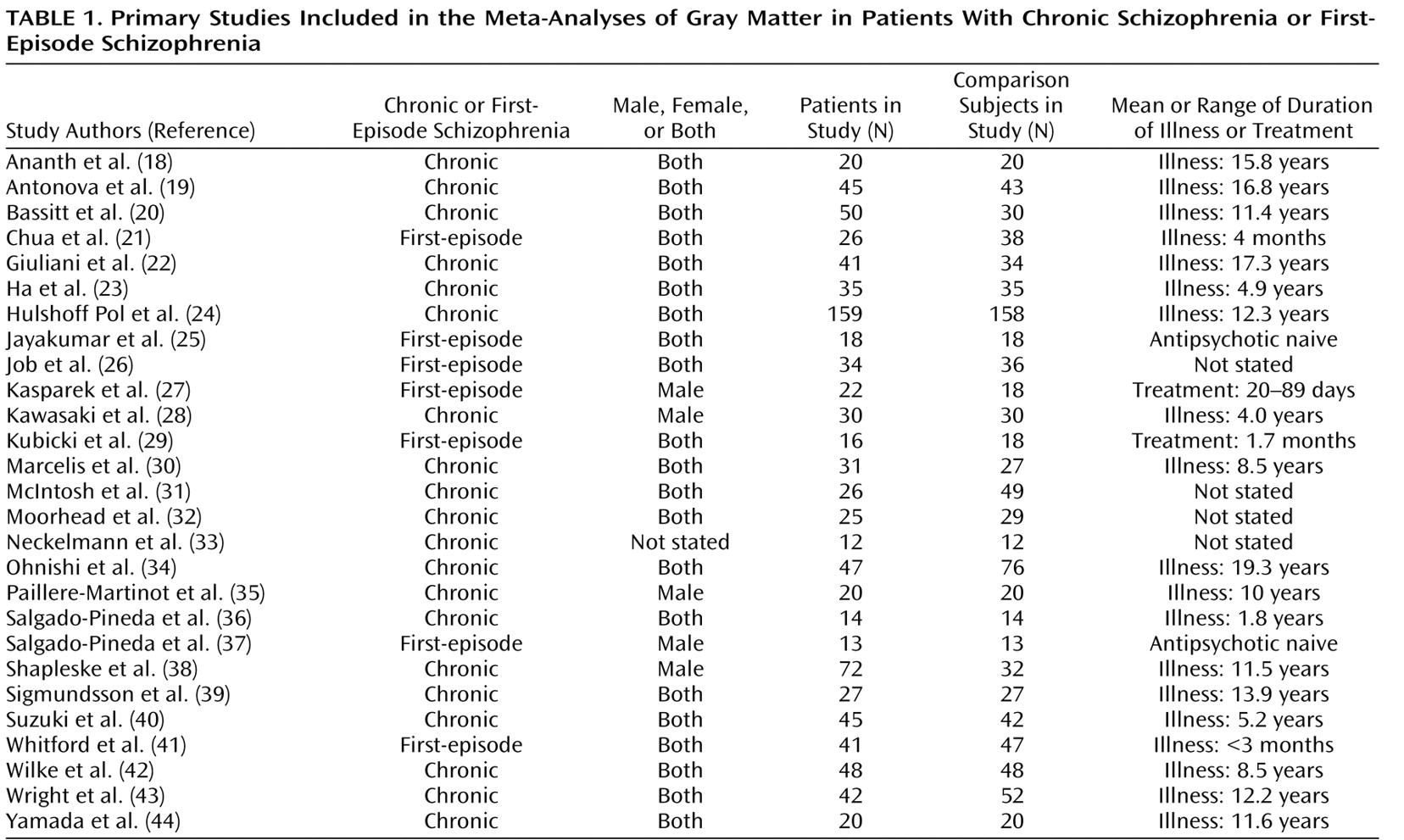
A total of 27 articles were identified for inclusion in the meta-analyses (
Table 1 ); the articles selected for each meta-analysis are listed in
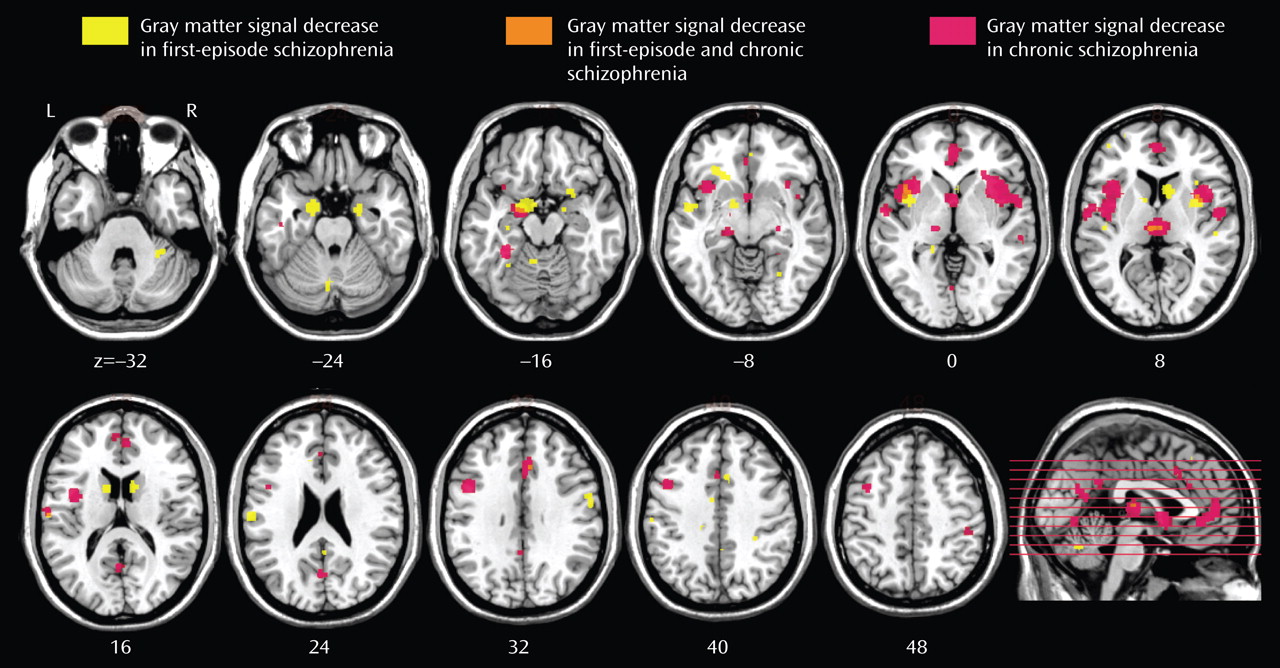
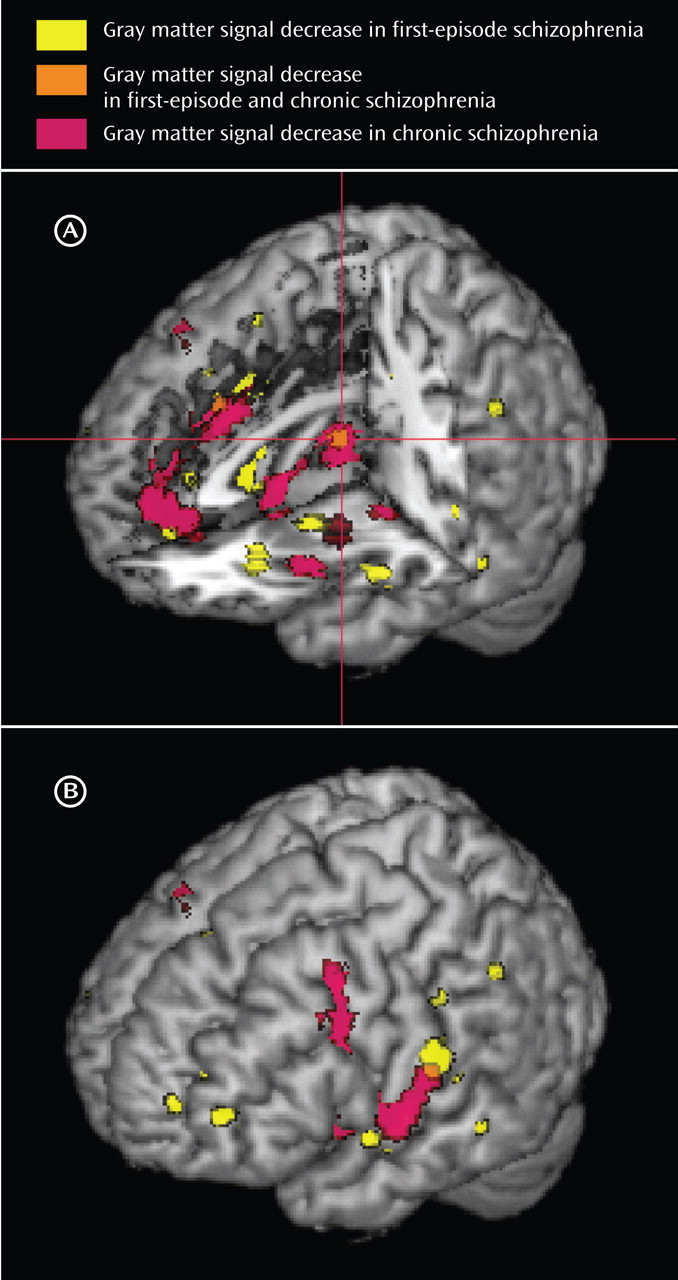
Table 2 . The clusters identified in each meta-analysis were obtained after controlling the false discovery rate at p<0.05 and applying a cluster extent threshold of 100 voxels. The gray matter decreases in first-episode schizophrenia and chronic schizophrenia are displayed on brain templates (
Figures 1 and
2 ) using the MRIcron software program (
49 ; www.sph.sc.edu/comd/rorden/mricron).
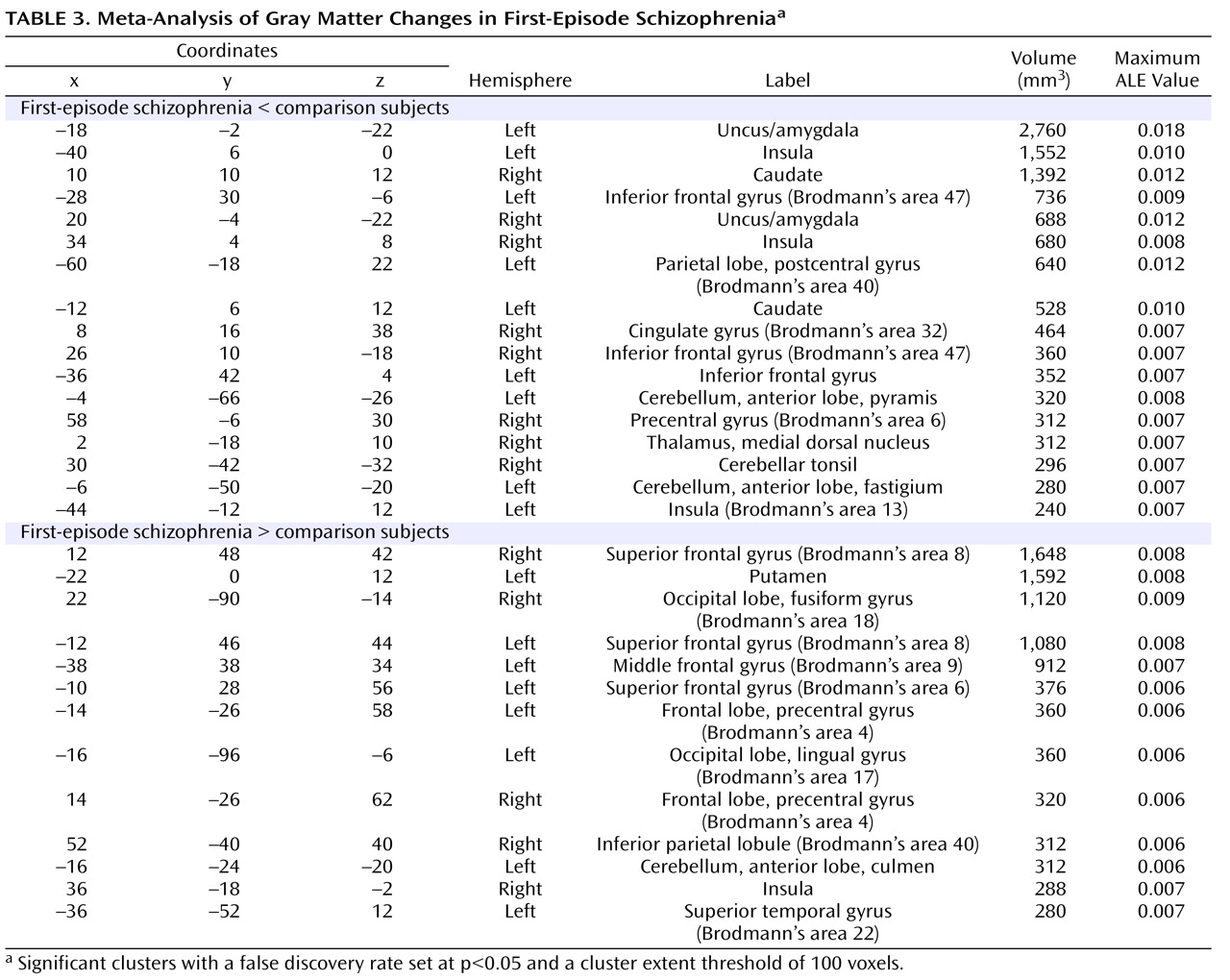
The coordinates for gray matter changes in first-episode schizophrenia are listed in
Table 3 . (For chronic schizophrenia and the comparison between first-episode schizophrenia and chronic schizophrenia, gray matter changes are listed in full in the data supplement that accompanies the online edition of this article.) The p values cited in the text represent the uncorrected voxelwise probability estimates for the maximum ALE value within each cluster, derived by permutation testing.
First-Episode Schizophrenia
There were gray matter decreases in subcortical structures: the caudate head (left side, p=0.0002; right side, p<0.0002) and the thalamus (medial dorsal nucleus region) (p=0.001). There were cortical gray matter decreases including the insula (left side, p<0.0002; right side, p=0.0002), the anterior cingulate gyrus (p=0.0016), and the inferior frontal gyrus (left side, p=0.0004; right side, p=0.001) and limbic gray matter decreases in the uncus/amygdala (left side, p<0.0002; right side, p<0.0002). There were also cerebellar gray matter reductions including the pyramis (p=0.0008).
There were gray matter increases including the left putamen (p<0.0002).
Chronic Schizophrenia
There were gray matter decreases in the thalamus (medial dorsal nucleus region) (p<0.0002). There were cortical gray matter decreases including the insula (bilaterally) (p<0.0002), the anterior cingulate gyrus (p<0.0002), the left inferior frontal gyrus (p<0.0002), the left middle frontal gyrus (p<0.0002), the left temporal fusiform gyrus (p<0.0002), and the right superior/middle temporal gyrus (p=0.0018). There were limbic gray matter decreases including the left uncus/amygdala region (p<0.0002) and the right hippocampus region (p=0.0004).
There were gray matter increases including the right putamen (p<0.0002) and the left putamen (p=0.0002).
Comparison of Gray Matter Decreases Between First-Episode Schizophrenia and Chronic Schizophrenia
Gray matter decreases were greater in first-episode schizophrenia in the caudate head bilaterally (p<0.0002) and in the left uncus (p<0.0002).
Gray matter decreases were greater in chronic schizophrenia in the medial frontal gyrus (p=0.0004) and the left dorsolateral prefrontal cortex (p<0.0002). In cortical regions (frontal, parietal, temporal, occipital, and insular cortex) as opposed to limbic (uncus, amygdala), basal ganglia, and cerebellar regions, gray matter decreases were more extensive in chronic schizophrenia (4,400 voxels) than in first-episode schizophrenia (2,200 voxels).
Discussion
These meta-analyses have identified a network of gray matter changes in first-episode schizophrenia. The changes were more extensive than previously suggested by meta-analyses of region-of-interest studies that identified reduced whole brain volume and hippocampal volume and increased ventricular volume in first-episode schizophrenia
(1,
2) .
In addition, we observed considerable overlap in the regions affected in both first-episode schizophrenia and chronic schizophrenia. In both groups, we found gray matter decreases in the thalamus, the left uncus/amygdala region, the left and right insulae, the anterior cingulate, and the left inferior frontal gyrus.
There are some potential limitations of the ALE technique. The technique does not differentiate between the different voxel heights (z-values) and spatial extents of foci in primary studies or between different group sizes and probability threshold values of primary studies
(50) . Many factors can influence the number of foci reported in primary studies. However, pooling multiple studies may be limited by such differences until rigorous standards of data reporting are developed for primary studies
(11) . Another potential source of bias in primary studies is the analysis of data using small-volume correction in which a subvolume of the brain is tested for statistical changes. This may lead to findings of more positive results in selected brain regions. In our meta-analyses, although we included coordinates derived using small-volume correction, these only contributed to 6% of the total coordinates, and repeating our analyses with these coordinates excluded did not materially affect the results.
Thalamocorticostriatal Circuits and Schizophrenia
The network of changes identified by these meta-analyses included regions that have previously been strongly implicated in the pathology of schizophrenia. Meta-analyses of region-of-interest studies (of patients mainly with chronic schizophrenia) have identified volume reductions in the thalamus
(5,
51), the anterior cingulate
(52), and the hippocampus
(53) . Region-of-interest studies in first-episode psychosis have also found reduced thalamic volume in some
(54 –
56) but not all
(57) studies. A study of the anterior cingulate cortex in first-episode schizophrenia identified reduced cortical thickness
(58) but not reduced gray matter volume.
Anatomically, the thalamus receives neural connections from the striatum (ventral striatum, caudate, putamen, and globus pallidus) and sends projections to (among others) the prefrontal and cingulate cortex, the hippocampus, and the limbic midbrain
(59) . The striatum (especially the ventral striatum) receives topographical glutamatergic cortical (prefrontal, temporal cortex) and limbic input as well as midbrain dopaminergic input (which appears to modulate the responses of the striatum to cortical and thalamic afferent input). Therefore, there is an anatomical substrate for a neural circuit linking the thalamus, prefrontal cortex, limbic regions, and striatum. Functional neuroimaging studies suggest that executive functioning deficits in schizophrenia may be mediated by basal ganglia-thalamocortical circuitry disruptions
(60) .
The “missing link” in this network is an anatomical change in the striatum. In first-episode schizophrenia, region-of-interest studies have found volume reductions in the caudate nucleus in some
(61) but not all
(54,
62,
63) studies. In this meta-analysis, we found decreases in gray matter in the caudate head (bilaterally) in first-episode schizophrenia, and these decreases were significantly greater than in chronic schizophrenia. Caudate volume deficits have been found in the offspring of patients with schizophrenia
(64), which may suggest a genetic contribution to this change.
Anatomical Differences Between the First-Episode and Chronic Patient Groups
We also investigated whether there were more extensive cortical changes in chronic schizophrenia compared with first-episode schizophrenia. We found that gray matter decreases were greater in chronic schizophrenia in the frontal cortex (medial frontal gyrus and left dorsolateral prefrontal cortex), the right insula cortex, and the left and right temporal cortex. These differences remained after correction for the number of available studies for chronic schizophrenia and first-episode schizophrenia by using a matched number of coordinates. Thus, although we did not find evidence for the temporolimbic progression of pathology from hippocampus to amygdala
(3), there was evidence for progression of cortical changes. Cortical gray matter deficits could arise from pathological disease progression, the effect of treatment
(65), or comorbidity
(66) .
Dopaminergic Pathways and the Role of Antipsychotic Medication
In both first-episode schizophrenia and chronic schizophrenia, there were regions of gray matter increases. In voxel-based morphometry, an “increase” may reflect a relative preservation from deficit (which may be detected in the context of whole brain gray matter volume loss), or it may represent an absolute increase in gray matter.
In first-episode schizophrenia there were gray matter increases including the left putamen, and in chronic schizophrenia there were gray matter increases including the putamen bilaterally.
The regions in this study that showed gray matter changes in schizophrenia were in the distribution of dopaminergic effects (nigrostriatal, mesolimbic, and mesocortical pathways). The dopamine system is one target for antipsychotic drugs that may alter brain structure
(67) . Antipsychotic treatment modulates putamen volume
(63,
68) and therefore may contribute to putamen volume increases in chronic schizophrenia. The brain changes in chronic schizophrenia may depend on whether patients receive treatment with conventional or atypical antipsychotic drugs, since conventional antipsychotics may be particularly associated with enlargement of the putamen
(69) and whole brain gray matter reductions
(65), particularly in some cortical areas
(69) .
A further implication of this study is that the caudate head gray matter deficit (identified in first-episode schizophrenia) “normalizes” as the course of schizophrenia becomes more chronic. If treatment with conventional antipsychotics results in gray matter volume increases in the basal ganglia
(68), then this may provide an iatrogenic mechanism for this effect.
The identification of these regions of gray matter changes in first-episode schizophrenia may assist in earlier diagnosis. Multivariate voxel-based morphometry has been used to differentiate patients with chronic schizophrenia from comparison subjects
(28) . Voxel-based morphometry has been used to study progression of brain changes in subjects at high risk of developing schizophrenia
(8) and has identified gray matter reductions over time that included the left uncus and the left inferior frontal gyrus (regions of change that this meta-analysis identified in first-episode schizophrenia). These regional changes had predictive value in identifying the subjects who went on to develop schizophrenia
(70) . The anatomical changes identified in our meta-analysis warrant further study as potential disease markers. Improved diagnostic specificity may be achieved by multivariate techniques
(71) or by shape analysis
(72), for example of the caudate head or uncus/amygdala.
In summary, these meta-analyses identified a distributed network of anatomical changes in first-episode schizophrenia. The pattern of results is consistent with theoretical models implicating thalamocorticostriatal circuits in the pathophysiology of schizophrenia. The network includes bilateral caudate head gray matter reductions, which are absent in chronic schizophrenia. There was also evidence for decreased frontal, insular, and temporal cortical gray matter in chronic schizophrenia, which is compatible with hypotheses of progressive anatomical abnormality over the course of the disorder.