Major depressive disorder in children and adolescents is a common, impairing, frequently comorbid condition; it is associated with problems in family and social functioning, difficulties in school performance, and an increased risk of recurrence, substance abuse, and suicidality
(1) . Effective treatment may reduce the impact of depression on psychosocial functioning and may lessen the risk of other adverse psychiatric sequelae. We previously reported the results of a meta-analysis of 27 randomized controlled trials of pediatric major depressive disorder, obsessive-compulsive disorder (OCD), and non-OCD anxiety disorders, presenting evidence that the benefits of antidepressants appear to be much greater than risks of suicidal ideation and suicide attempt across indications
(2) . The efficacy of antidepressant treatment was greatest for non-OCD anxiety disorders, intermediate for OCD, and more modest for major depressive disorder, with numbers needed to treat of 3, 6, and 10, respectively. However, while the response to antidepressants was similar across diagnostic groups (range=46% to 63%), the main difference was that the response to placebo was higher for youths with major depressive disorder (50%) when compared with the placebo response for youths with OCD and non-OCD anxiety disorders (32% and 39%, respectively). Furthermore, in major depression trials, the placebo response rate appeared to be higher in studies with greater numbers of sites and in children under age 12 compared with adolescents.
Identification of the characteristics of youths most likely to respond to placebo could be useful for both research and clinical care. In research, youths who are highly likely to respond to placebo could be screened out, which might decrease the necessary sample size and increase the power of treatment trials to detect differences between active treatment and placebo. In clinical care, there are acute shortages of therapists trained in one of the indicated psychotherapies and of physicians skilled in the use of antidepressants
(3,
4) . Identification of those children least likely to respond to placebo could help to prioritize referrals. Finally, while the reported risks of selective serotonin reuptake inhibitors (SSRIs) appear to be outweighed by the benefits, this would clearly not be the case in someone who is just as likely to respond to placebo. Therefore, identification of youths who are likely to respond to placebo would substantially increase the benefit-to-risk ratio for the use of antidepressants in pediatric depression. Moreover, depressed youths who are likely to respond to placebo could be offered a brief psychosocial intervention rather than medication or other more intense types of psychotherapies
(1,
5,
6) .
While the response to placebo in depressed youths has not been well studied
(5), predictors of higher placebo response rates in depressed adults have been identified, including younger age, male sex, longer duration of the antidepressant trial, shorter duration of illness, lower severity of depression, fewer prior episodes, and study location (with higher placebo response in major depression trials conducted in Europe compared with the United States)
(7 –
12) .
In this study, we used summary data from all available published and unpublished trial reports to examine patient and methodological factors that may predict placebo response in pediatric depression. Secondary aims included quantifying the magnitude of response to active medication that can be explained by placebo response and the impact of placebo response on the drug-placebo difference in efficacy
(13), and determining whether the proportion of pediatric patients with major depression responding to placebo has increased in recent years, as has been reported for adults in a study of outpatients with major depression
(14) .
Results
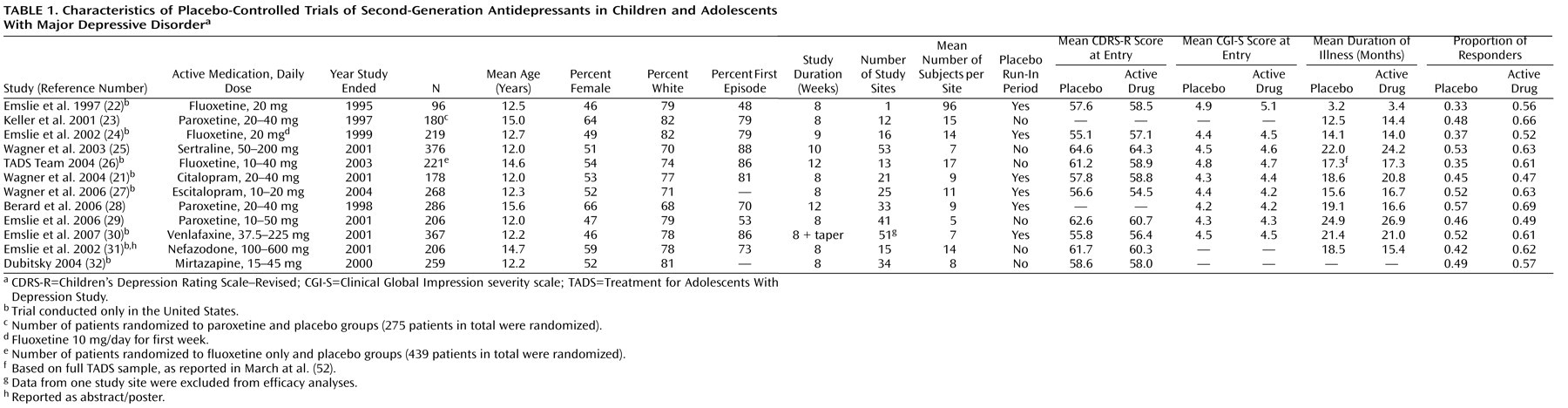
In the 12 studies we analyzed, 2,862 patients underwent randomized assignment to active medication or placebo (
Table 1 ). The median age was 12.3 years (range=12.0–15.6 years), and the median proportion of female participants was 0.53 (range=0.46–0.66). The mean proportion of participants per study responding to placebo was 0.46 (SD=0.08, range=0.33–0.57), and for active drugs, 0.59 (SD=0.07, range=0.47–0.69).
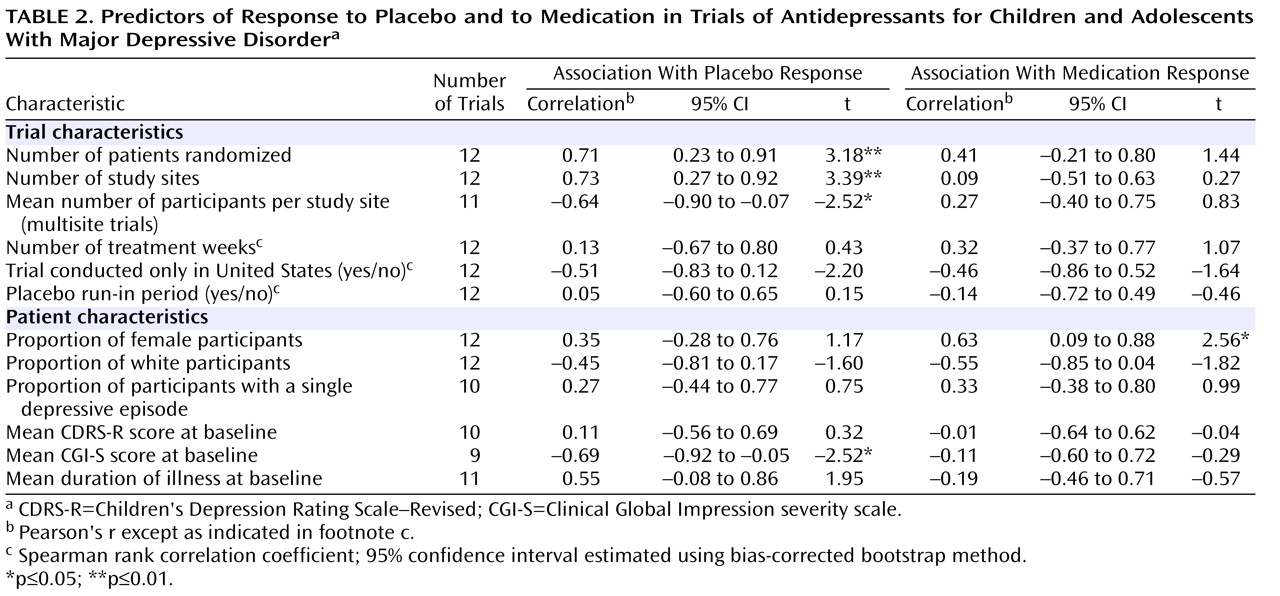
As shown in
Table 2, there were significant correlations between proportion of placebo responders and each of the three site selection variables—number of patients who underwent randomized assignment, number of study sites, and average number of participants per study site.
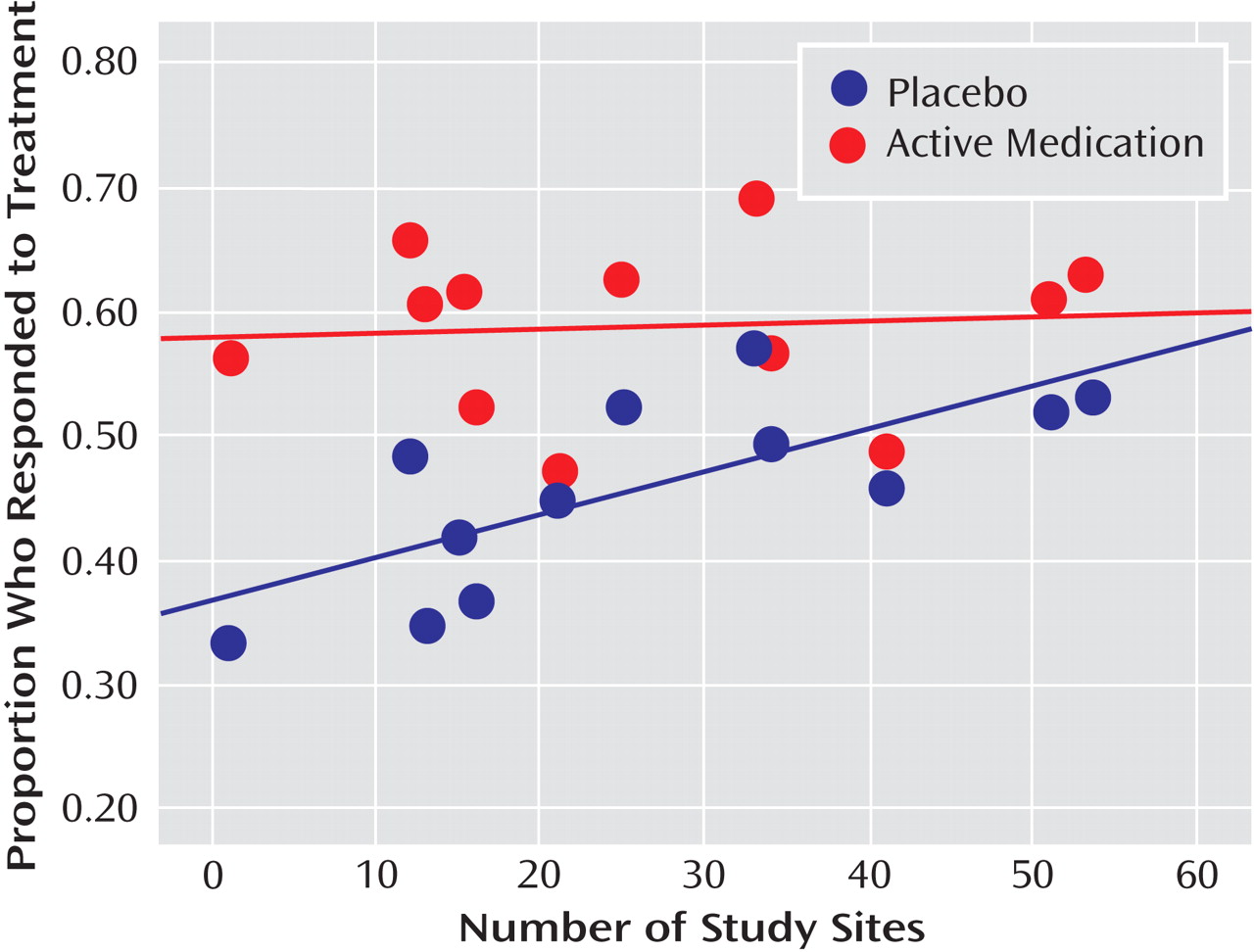
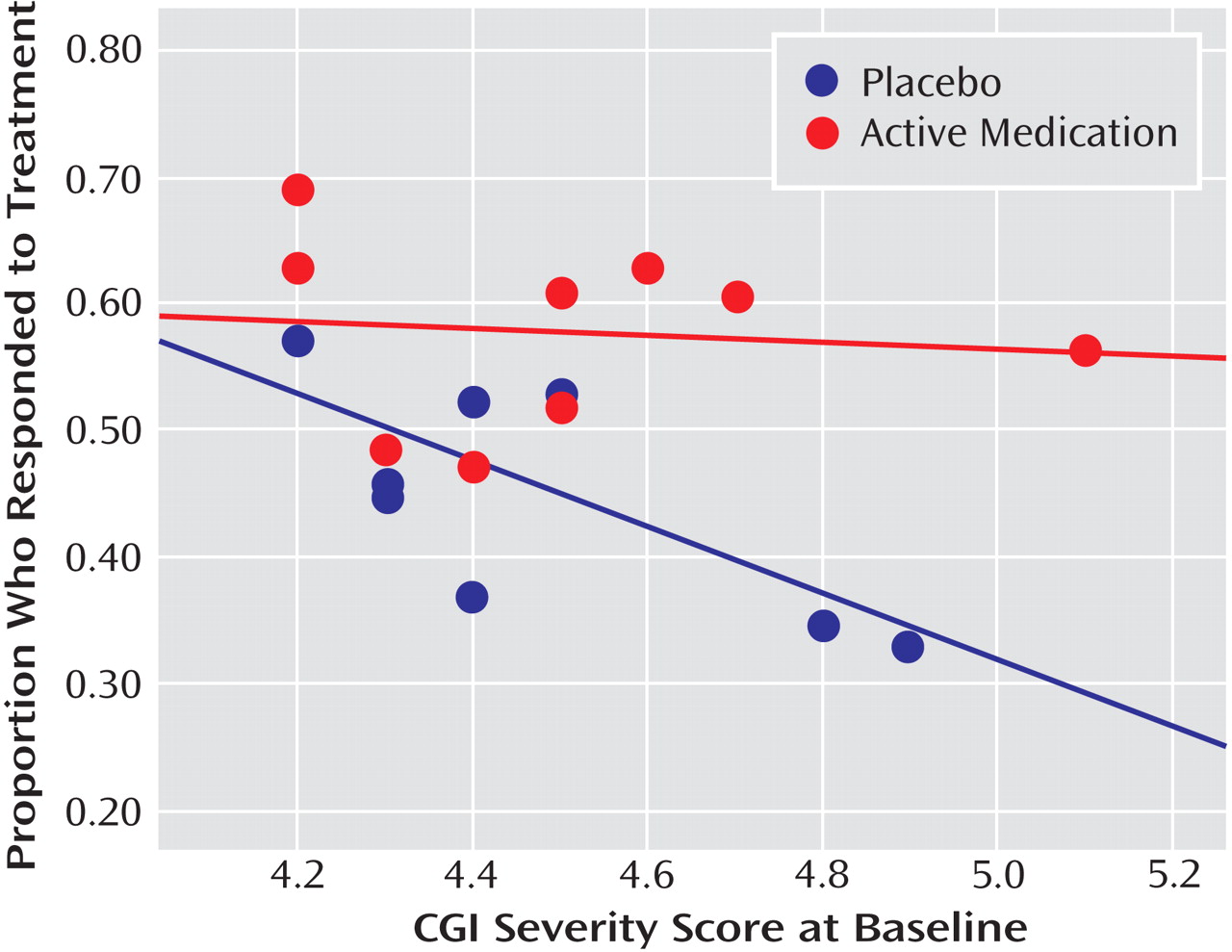
Figure 1 plots the proportion of placebo responders by number of study sites. Severity of illness at entry, as assessed by the CGI severity item, showed an inverse relationship with placebo response, indicating a higher placebo response with decreasing severity (
Figure 2 ). Sensitivity analyses confirmed that these significant associations were generally insensitive to the exclusion of any single trial (number of patients randomized, r range: 0.59 to 0.76; number of study sites, r range: 0.64 to 0.79; average number of participants per site, r range: –0.48 to –0.75; CGI severity score, r range: –0.54 to –0.80). No correlations were evident between proportion of placebo responders and duration of the treatment period, duration of illness, study location, placebo run-in period, CDRS-R score, or proportion of female participants, white participants, or participants who had first-episode major depressive disorder. The predictors for response to placebo and response to active medication were distinct. Among the above-noted variables, only the proportion of participants who were female was positively correlated with antidepressant response.
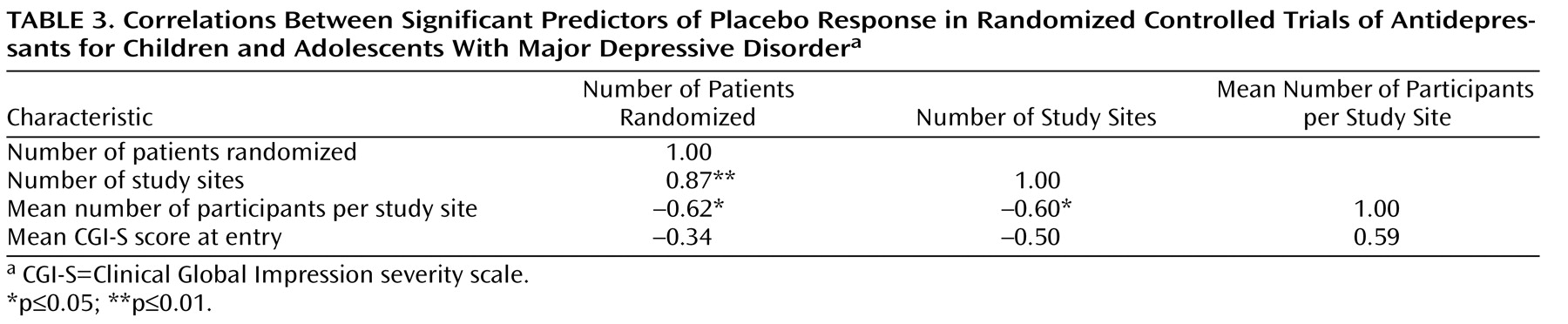
All three site selection variables were intercorrelated (
Table 3 ). In order to explore further the factors most associated with placebo response, we conducted a multiple linear regression analysis using a logit transformation of the proportion of placebo responders per study as the dependent variable and CGI severity score and number of study sites as predictor variables; number of study sites was selected for analysis because it was the only site selection variable to survive both a backward-stepping multiple regression analysis with control for other site selection variables and to enter a forward-stepping regression model predicting proportion of placebo response (data available on request). In the multiple regression analysis with control for number of study sites and initial severity, only number of study sites remained significant (β=0.59, t=2.46, df=6, p=0.05, partial r=0.71); severity of illness did not reach statistical significance (β=–0.40, t=1.69, df=6, p=0.14, partial r=–0.57), although the adjusted effect size for initial severity can be considered large
(36) .
In the nine trials for which age-grouped data were available
(22 –
26,
28 –
30,
37), the rate of placebo response was not significantly different between children and adolescents (49.6% [95% CI=43.7–55.5] and 44.5% [95% CI=40.9–48.2%], respectively). (See the figure in the data supplement that accompanies the online edition of this article.) Systematically deleting one study at a time revealed that the overall results were substantively altered by excluding one fluoxetine trial
(24), yielding placebo response rates of 54.3% (95% CI=47.7–60.9) in children and 44.9% (95% CI=41.1–48.7) in adolescents (χ
2 =5.87, p=0.02). The rate of response to active medication was not significantly different between children and adolescents (58.4% [95% CI=52.5–64.3] and 61.5% [95% CI=58.2–64.8], respectively), and the exclusion of any single trial from the analysis did not substantively change the overall result.
The magnitude of placebo response appeared to play a larger role in predicting the drug-placebo difference in efficacy than did the magnitude of active medication response, despite a nonsignificant correlation between response to placebo and response to active medication (N=12; r=0.47; 95% CI=–0.14 to 0.82, p=0.13). The proportion of patients responding to placebo was strongly related to both the risk difference in CGI improvement response (N=12; r=–0.61; 95% CI=–0.88 to –0.06, p=0.03) and to the scalar change in symptoms from baseline to end of treatment in active medication versus placebo (Hedges’ g, N=12; r=–0.78; 95% CI=–0.93 to –0.37, p=0.003); however, neither the association with risk difference in response nor Hedges’ g was significant after correcting for number of sites. On the other hand, the proportion of responders to active medication was not related to either of these measures of efficacy (risk difference, N=12; r=0.41; 95% CI=–0.21 to 0.79, p=0.18; Hedges’ g, N=12; r=–0.18; 95% CI=–0.68 to 0.44, p=0.58).
Studies included in this report were published between 1997 and 2007. The proportion of patients responding to placebo was significantly correlated with year of publication (N=10; r=0.64; 95% CI=0.02 to 0.91, p=0.05), indicating an increase in placebo response over time. The number of study sites increased significantly with year of publication (N=10, r=0.69, p=0.03); in multiple linear regression analyses, neither year of publication nor number of study sites was a significant predictor of the proportion of patients responding to placebo. Because the nefazodone and mirtazapine trials
(31,
32) were unpublished, we also examined the association between treatment response and year of study completion, which occurred between 1995 and 2004. The association between year of study completion and placebo response was not statistically significant, suggesting that the increase in placebo response over time is due to a publication artifact. The average time between year of study completion and year of publication for the five trials that found evidence of efficacy for antidepressants
(21,
22,
24 –
26) was around 2 years (mean=28 months [SD=10]), compared with an average of almost 5 years to publication for negative trials (mean=59 months [SD=25]; p=0.03). No significant correlations were found between the proportion of patients responding to active medication and year of publication or year of study completion.
Discussion
In this study of all available reports of randomized clinical trials of antidepressant treatment for pediatric major depression, we found that the proportion of participants responding to placebo, but not the proportion responding to active medication, was strongly related to the number of study sites. Initial severity of illness was inversely related to the proportion of patients responding to placebo, although this association was reduced when number of sites was controlled for. The proportion of placebo response may also be higher in younger participants, as we found after excluding one trial of fluoxetine that showed a low rate of placebo response across the age range of participants
(22) . While the placebo and active medication responses were intercorrelated within studies, the placebo response explained more of the variance in efficacy than did the response to active medication. Consistent with previous meta-analyses, the placebo response rate was higher in more recently published studies, although this effect was explained in part by a change in study characteristics over time and a publication bias. Before we discuss each of these findings, we first place the results of this study within the context of its limitations.
This study is restricted to an analysis of trial-level summary data, which may fail to identify important individual patient factors influencing the response to placebo
(38) . In addition, even trial-level data were inconsistently reported, and thus we were unable to examine the impact of several other factors that have been shown to be relevant to clinical response and presumably are relevant to placebo response, such as history of nonresponse to an SSRI, history of abuse, family history of psychiatric disorder, and comorbid psychiatric disorders
(23,
39 –
42) . Because the relatively small number of studies gave us low statistical power, statistical interactions were not examined, and null findings should be interpreted with caution. Finally, participants in clinical trials may differ from real-world clinical populations, which may limit generalizability
(43) .
The number of study sites proved to be the strongest predictor of placebo response (but not response to active medication), which expands on our earlier finding that the magnitude of antidepressant treatment efficacy decreased as the number of study sites increased
(2) . The correlation between mean study severity and number of sites (r=–0.50) indicates that studies with many sites recruited participants with less severe illness, suggesting that screening for participants may be less stringent in trials with more sites. When possible, limiting the number of investigative sites to a few larger centers with tighter control may improve the ability to screen out likely placebo responders. Development of a treatment research network of a small number of sites that are experienced in clinical research for child and adolescent mood disorders might provide an optimal strategy for assessing the relative short-term and long-term efficacy of pharmacological and nonpharmacological treatments. Alternatively, when there is a need for many sites, screening carefully for illness severity is essential. Given that site differences have been shown to increase the likelihood of negative or failed antidepressant trials in adults, efforts to reduce potential sources of variability (e.g., amount of investigator experience, procedures used to recruit subjects) within and between study sites are needed
(44) .
Consistent with a recent meta-analysis
(12), a higher proportion of participants responding to placebo but not to antidepressants was predicted by decreased baseline CGI severity. These results should be interpreted with caution because three trials did not assess severity using CGI criteria and were excluded from analyses. If confirmed, these findings raise questions about the benefit-to-risk profile of antidepressants in treating depressed pediatric patients with mild functional impairment. It is unclear why CDRS-R symptom scores did not correlate with placebo response when we found a strong relationship between the CGI severity score and placebo response on univariate analysis. However, at a trial level, the low correlation between the mean baseline CDRS-R and mean CGI severity score (r=0.05, p=0.91) suggests that these two rating scales are measuring different aspects of illness severity. Moreover, while the majority of studies (10/12) had a minimum CDRS-R entry requirement, only three of the trials used a cutoff on the CGI severity score. This selection strategy may explain the high placebo response, since the CGI severity score is a stronger correlate of placebo response than is baseline CDRS-R. From a clinical perspective, these data suggest a role for supportive therapy or a brief (4- to 6-week) trial of more specialized psychotherapy as first-line treatment options for mild depression
(1,
5,
45) .
We found no significant overall age effect in placebo response, but a sensitivity analysis that excluded one fluoxetine trial revealed that children younger than age 12 had a higher placebo response rate than adolescents age 12 and older. This finding is consistent with our previous report
(2) showing that the lack of a significant treatment effect for antidepressants other than fluoxetine in children may be due in part to a higher placebo response in children than in adolescents. However, a recent post hoc analysis of two fluoxetine trials
(22,
24) showed a vigorous antidepressant response and lower placebo response in children under age 12 compared with adolescents
(46) . Thus, it appears premature to conclude that the placebo response rate is higher in children than in adolescents.
Despite some covariation between response to medication and to placebo in this study (r=0.47), higher placebo response emerged as a potent negative correlate of treatment efficacy, an association that was attenuated when number of study sites was controlled for. Conversely, in some trials, it was clearly possible to recruit depressed individuals for whom a placebo intervention was not very efficacious, and not surprisingly, it was in these studies that the effects of antidepressants were most robust
(22,
26) . Patient-level regression tree analyses that use participants’ demographic and clinical characteristics to develop models of response prediction may offer one promising approach for future work aimed at identifying candidate variables associated with optimum drug response on the one hand and the lowest placebo response on the other
(47 –
49) .
In published placebo-controlled trials of adult depression, response rates increased between 1980 and 2000 among both placebo and active medication groups
(14) . We found that the reported relationship between placebo response and date of publication was actually explained by the increasing trend over time toward large multisite trials and an artifact of publication bias. When the date of study completion was taken into account, there was no relationship between the proportion of participants responding to placebo and the temporal order of study completion, which highlights the need for prompt reporting of study results regardless of outcome
(50) . A recent study found that selective reporting of adult antidepressant medication results exaggerated their effectiveness, which could mislead physicians and patients about the relative efficacy of antidepressant treatment
(51) .
In summary, our findings indicate that the placebo response in controlled antidepressant trials of pediatric depression is strongly correlated with the number of study sites. Restricting the number of sites to a few select centers with experience in clinical assessment of pediatric mood disorders may allow for more careful selection of participants and improved quality assurance over the methods individual sites use to approach, recruit, and retain the patients under study. Since many young patients with an episode of mild depression may respond to brief supportive therapy
(5,
6,
52), future work should aim to identify the level of clinical severity at which first-line treatments with medication, psychotherapy, or their combination are warranted.