While placebo-controlled trials are necessary to establish the efficacy and safety of medications, concerns have been raised about the long-term impact of delaying active treatment with use of a placebo
(1) . Khan and colleagues
(2), in a meta-analysis of adult antidepressant trials, determined that individuals treated with placebo received adequate care. Patients who received placebo experienced substantial symptom reduction, although of a lesser magnitude than those in active treatments, and differences between drug and placebo outcomes decreased as study duration increased. Furthermore, the placebo-treated patients received all other active components of treatment, including a thorough evaluation, psychoeducation about depression, access to a treatment provider, supportive care, and the expectation of improvement
(2) .
There have been far fewer placebo-controlled trials of antidepressants in children and adolescents than in adults, and only about one-fourth of them have demonstrated superiority of active drug over placebo
(3) . While the use of placebo is frequently challenged as unethical and unnecessary, researchers and bioethicists often regard the inclusion of a placebo control as useful in attaining certain scientific goals of clinical trials. For example, placebo trials provide the ability to contrast adverse events associated with active treatment, helping to differentiate the baseline rate of physical and psychiatric symptoms that occur routinely in individuals from symptoms due to study intervention. Additionally, the use of a placebo control can allow for reasonable assay sensitivity in smaller studies, resulting in lower cost, shorter study duration, and fewer subjects exposed to a potentially ineffective or poorly tolerated treatment.
Some of the factors to consider when weighing the appropriateness of a placebo in a trial include the availability of effective alternatives to the study interventions, the stability of the psychopathology to be treated, the potential for direct benefit from placebo, the use of rescue procedures for placebo patients whose clinical situation deteriorates, and the toxicity of the study intervention. However, it is also important to be aware of when a placebo control may be inappropriate. For example, inclusion of a placebo is not appropriate in situations where significant harm may result from deferring therapies known to be beneficial
(4,
5) . Little is known about the long-term outcomes of acute treatment with placebo in pediatric antidepressant trials. Furthermore, there have been no reports describing longitudinal monitoring of suicidal ideation or suicide attempts, severe adverse events, or adjunctive service utilization among depressed youths initially exposed to placebo.
A recent research forum sponsored by the American Academy of Child and Adolescent Psychiatry cited several key elements to consider in deciding whether placebo is an acceptable control condition, including the potential for direct benefit from placebo, limiting the risk of harm from withholding active treatment, and use of rescue procedures to minimize negative consequences
(5) . In this study, using these three key elements, we evaluated the longer-term consequences, including benefits and risk of harm, of being assigned to 12 weeks of placebo treatment followed by open active treatment.
The Treatment for Adolescents With Depression Study (TADS), a randomized controlled trial comparing pharmacotherapy with fluoxetine, cognitive-behavioral therapy (CBT), combination treatment with fluoxetine and CBT, or clinical management with pill placebo in adolescents with major depressive disorder, has been described in previous reports
(6 –
9) . The 12-week placebo response rates and safety outcomes for those assigned to placebo have been described elsewhere
(7,
10) . The TADS design, which included consolidation and maintenance phase treatment and assessments, allows for investigation of the longer-term outcomes of participants treated with 12 weeks of placebo followed by open active treatment.
Method
Study Participants and Treatment
A total of 439 participants 12–17 years of age were randomly assigned to one of four treatment conditions: fluoxetine (N=109), CBT (N=111), combination treatment with fluoxetine and CBT (N=107), and placebo (N=112). In this article, we refer to those assigned to placebo as the “placebo/open” group to reflect the fact that these patients were entered into open-label treatment and were actively treated as clinically indicated after acute treatment. We compared the placebo/open group to all active treatment groups combined.
TADS treatment consisted of three stages: stage 1, the acute phase, lasting 12 weeks; stage 2, the consolidation phase, lasting 6 weeks; and stage 3, the maintenance phase, lasting 18 weeks. In stage 1, patients in the placebo/open group had six visits with the study psychiatrist. Dosing for this group was matched to that of the fluoxetine group, with one placebo tablet for each 10-mg fluoxetine tablet, beginning with 1 week at 10 mg/day, followed by an increase to 20 mg/day. The dosage could be increased (based on clinician judgment) to 30 mg/day after 4 weeks or 40 mg/day after 6 weeks, to a maximum of 40 mg/day. At the end of stage 1, the blind was broken, and placebo/open participants who had not improved (that is, those who had a score >2 on the improvement item of the clinician-rated Clinical Global Impression scale [CGI;
11 ], indicating partial response or worse) were offered 12 weeks of their choice of open TADS treatment. Participants in the placebo/open group who responded to treatment were offered telephone follow-up over the next 3 months and were offered 12 weeks of their choice of TADS treatment if they relapsed (based on clinician judgment) during the follow-up period. Those in the active treatment groups who responded or had a partial response to treatment continued in their assigned treatment condition for stages 2 and 3.
Assessment and Measures
TADS assessments have been described in detail elsewhere
(7,
9) . Assessments were conducted by an independent evaluator at 6-week intervals. They were continued for all participants, regardless of continued participation in TADS treatment, through the 36-week study period.
Direct benefit
The Children’s Depression Rating Scale–Revised (CDRS-R)
(12) was completed at each assessment. This 17-item measure of depression severity, rated on 5- and 7-point scales, is an interviewer-rated scale of depressive symptoms compiled from separate interviews with adolescents and parents. Interrater reliability on the CDRS-R, assessed at baseline and at week 12, was found to be high (0.95 at baseline and 0.98 at week 12). The CGI improvement item is a 7-point interviewer-based Likert scale measuring improvement in depressive symptoms relative to baseline. Response to treatment was defined as a score of 1 or 2 on the CGI improvement item (indicating “very much improved” or “much improved”) at week 12. If the week 12 assessment was missed, the last-observation-carried-forward method was used to calculate response status. Partial response was defined as a score of 3 on the improvement item. Remission was defined as a CDRS-R score ≤28
(13) .
Logs of concomitant treatment and medications, as well as a modified version of the Child and Adolescent Services Assessment
(14), were used to document mental health treatment received outside of TADS. The Child and Adolescent Services Assessment is designed to collect data on mental health service utilization (defined as inpatient or outpatient services for emotional, behavioral, or substance use problems).
Risk of harm
Worsening of depression was defined as a CGI improvement item score of 5, 6, or 7 at any point from week 12 through week 36. For participants whose week 12 improvement item score was missing, the last-observation-carried-forward method was applied. Serious adverse events were coded using a definition of worsening of suicidality relative to baseline and using standard U.S. Food and Drug Administration language: life-threatening (immediate risk of death); requires hospitalization; or results in persistent or significant disability, incapacity, congenital anomaly, birth defect, death, or other significant medical event. Primary suicidal events were defined using the Columbia Classification Algorithm of Suicidal Assessment
(15) and coded independent of knowledge of treatment or course by the Columbia University Suicidality Classification group. A primary suicidal event was counted as occurring if the participant had any one of the following: suicide attempt, preparatory action toward suicidal behavior, or suicidal ideation.
Rescue procedures
Manual-based adjunct services for attrition prevention were provided by TADS clinicians to address clinical crises and retain participants
(16) . Rescue procedures were defined as the use of these adjunct services on at least one occasion during each assessment period.
Statistical Analyses
The primary analyses of response and remission were conducted using an intention-to-treat approach in which analyses included all participants who underwent random assignment regardless of protocol adherence, treatment completion, or treatment response at the end of acute treatment (stage 1). Analyses examined response and remission rates during stage 2 (weeks 12–18) and stage 3 (weeks 19–36) among participants assigned to placebo/open treatment or to active treatment. All primary analyses were adjusted for site.
General linear models and chi-square tests were used to compare baseline characteristics of youths in the placebo/open group with those in the active treatment groups. Because of the non-normality of data distributions, nonparametric Wilcoxon two-sample tests were used to compare duration of current major depressive episode and number of comorbid psychiatric disorders.
Generalized estimating equations compared treatment differences in response and remission rates and estimated the probabilities of remission at weeks 12–36 for all 439 participants. Each model tested for effects of treatment and time (assessment week) and their interaction, with site included as a covariate. The original 13 clinical sites were collapsed into 10 sites, with four low-enrollment sites of similar geographic or recruitment characteristics combined into a single site. When missing, week 12 data were imputed using the last-observation-carried-forward method (N=61: 12 in fluoxetine treatment, 21 in CBT, 12 in combination treatment, and 16 in placebo/open treatment). Chi-square testing or Fisher’s exact test was used to examine between-treatment differences in proportion of youths with suicidal events, serious adverse events, CGI improvement item worsening, study retention, and TADS and non-TADS interventions at various time points.
All analyses were conducted using SAS, version 8.2 (SAS Institute, Cary, N.C.), and the significance threshold was set at 0.05 (two-tailed) for each test. A posteriori paired treatment comparisons were conducted only if the omnibus test was significant at 0.05 for treatment or treatment by assessment week.
Results
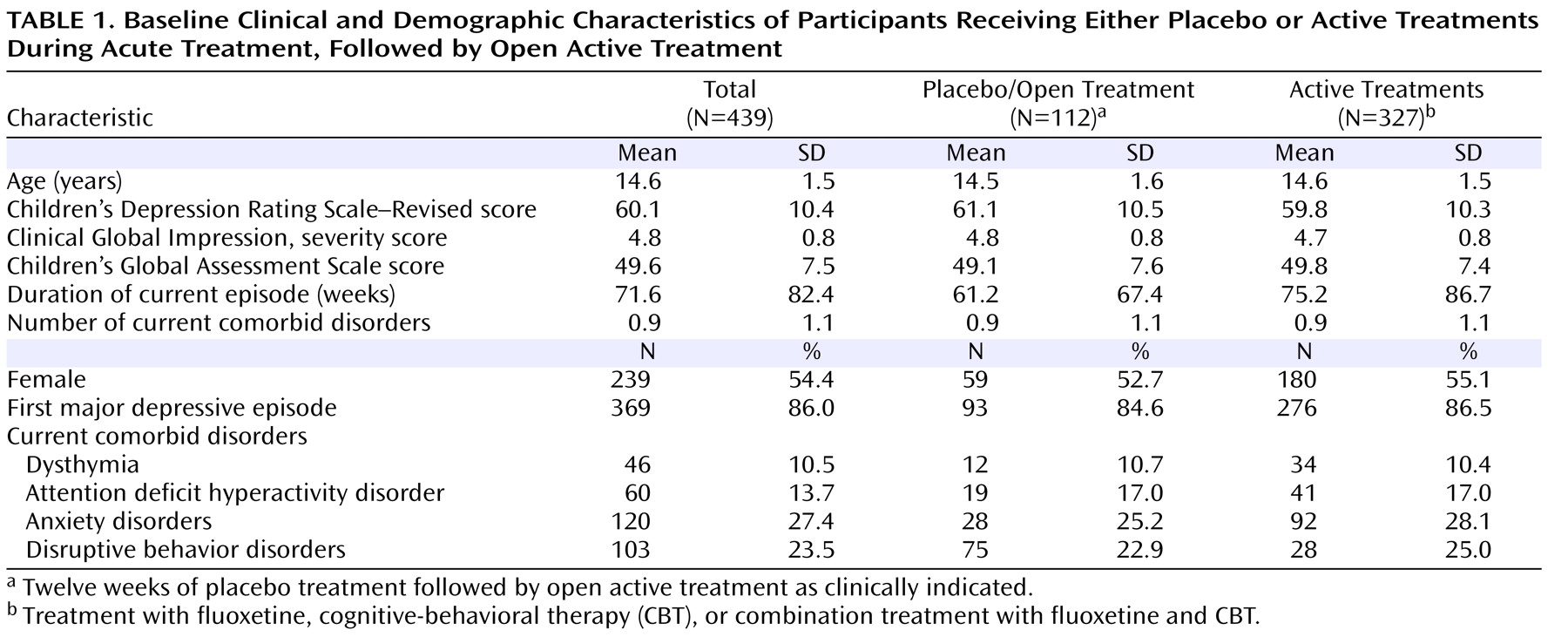
Baseline clinical and demographic characteristics for the groups are presented in
Table 1 . There were no statistically significant differences between the placebo/open and active treatment groups on baseline characteristics.
Flow of Patients in Placebo/Open and Active Treatment Groups
Sample maintenance
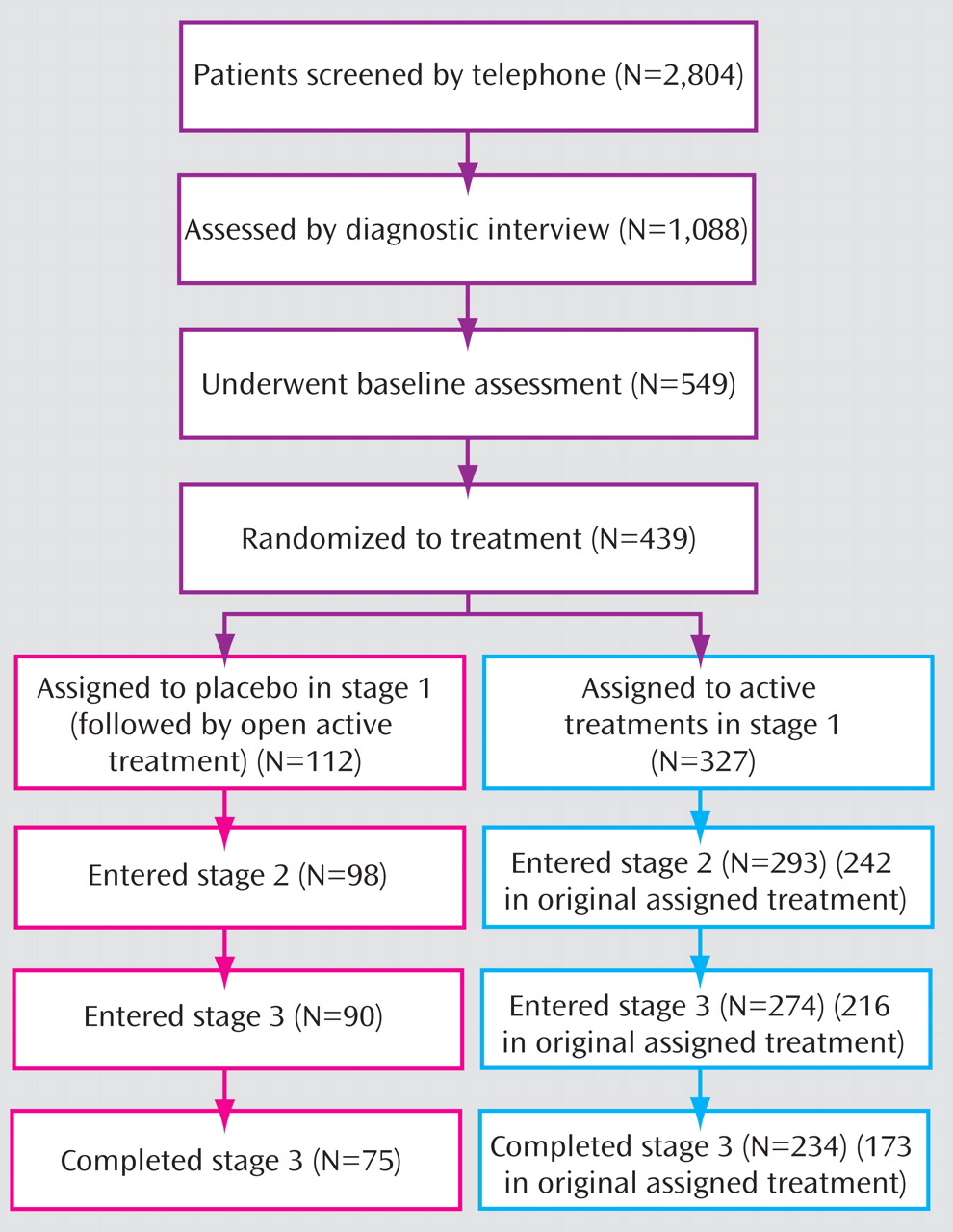
Figure 1 presents the CONSORT diagram of participants through the various stages of treatment. Note that for participants in the placebo/open group, in stages 2 and 3 treatment was not structured in terms of consolidation and maintenance. Within the 24 weeks of stages 2 and 3, these patients were offered 12 weeks of open TADS treatments and 12 weeks of uncontrolled continued care, although the study’s definitions of the stages were retained for analytical purposes.
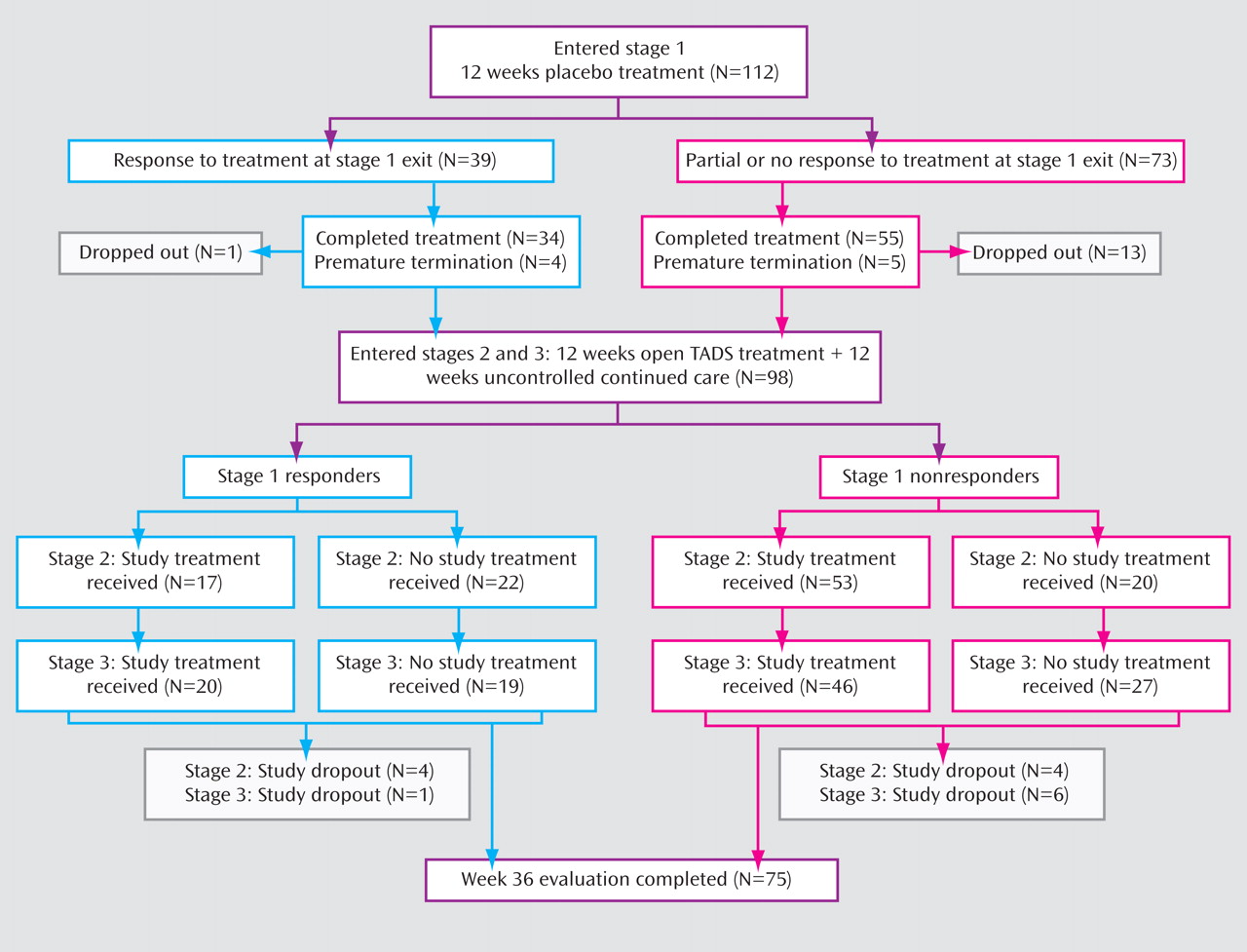
Figure 2 presents the flow of participants assigned to placebo/open treatment through the 36 weeks of the study. Of the 112 participants in this group, 98 (87.5%) continued their participation in stage 2, and 90 (80.4%) were still in the study at the beginning of stage 3. We compared the demographic and clinical characteristics of those in the placebo/open group who dropped out of the study during stages 1–3 and those who completed stage 3. Those who did not drop out were younger on average (mean age, 14.3 years [SD=1.6] compared with 15.1 years [SD=1.5]; z=2.32, p=0.02) and had a longer mean duration of current episode (68.4 weeks [SD=72.8] compared with 40.3 weeks [SD=43.4]; z=–2.27, p=0.02). There were no differences in dropout rates across the 36 weeks between the placebo/open and active treatment groups. We also compared dropout rates at week 36 between participants who responded by week 12 and those who did not, and found no differences between responders and nonresponders in the active treatment and placebo/open groups.
TADS treatment utilization by placebo/open participants in stages 1–3
During stage 1, 13 participants in the placebo/open group (11.6%) were prematurely terminated from the randomized treatment by the TADS clinician because of clinical worsening. Of those, five then received combination treatment, four received fluoxetine, and four dropped out of the study and did not receive any active TADS treatment during stage 1. One placebo/open participant received CBT while still active in the placebo condition (protocol deviation). Thus, 10 placebo/open participants (8.9%) received TADS active treatment in stage 1 (nine of them after prematurely terminating and one receiving CBT along with placebo).
Among the 73 placebo/open participants who did not respond during stage 1, 45.2% received combination treatment in stage 2, 24.7% received fluoxetine, 2.7% received CBT, and 27.4% elected not to receive treatment within TADS during stage 2. During stage 3, 39.7% received combination treatment, 16.4% received fluoxetine, 6.9% received CBT, and 37% chose not to receive TADS treatment. Among the 39 placebo/open participants who responded in stage 1, 5.4% received combination treatment in stage 2, 20.5% received fluoxetine, 7.7% received CBT within TADS, and 56.4% elected not to receive TADS treatment during stage 2. In stage 3, 12.8% received combination treatment, 25.6% received fluoxetine, 12.8% received CBT, and 48.7% elected not to receive TADS treatment.
Non-TADS treatment utilization among placebo/open participants
At week 12, no significant differences in receipt of non-TADS mental health treatment were found between placebo/open (5.3%; 5/94) and active treatment participants (7.3%; 20/273). Similarly, at week 24, there were no significant differences between groups, with 15.6% of the placebo/open (12/77) and 15.9% of the active treatment (37/233) participants using outside services. However, at week 36, the placebo/open group had more outside service utilization (25/73; 34.3%) than the active treatment group (40/221; 18.1%; χ 2 =8.31, p<0.004).
Potential for Direct Benefit From Placebo
CGI improvement item response rates for placebo/open participants in stages 2–3
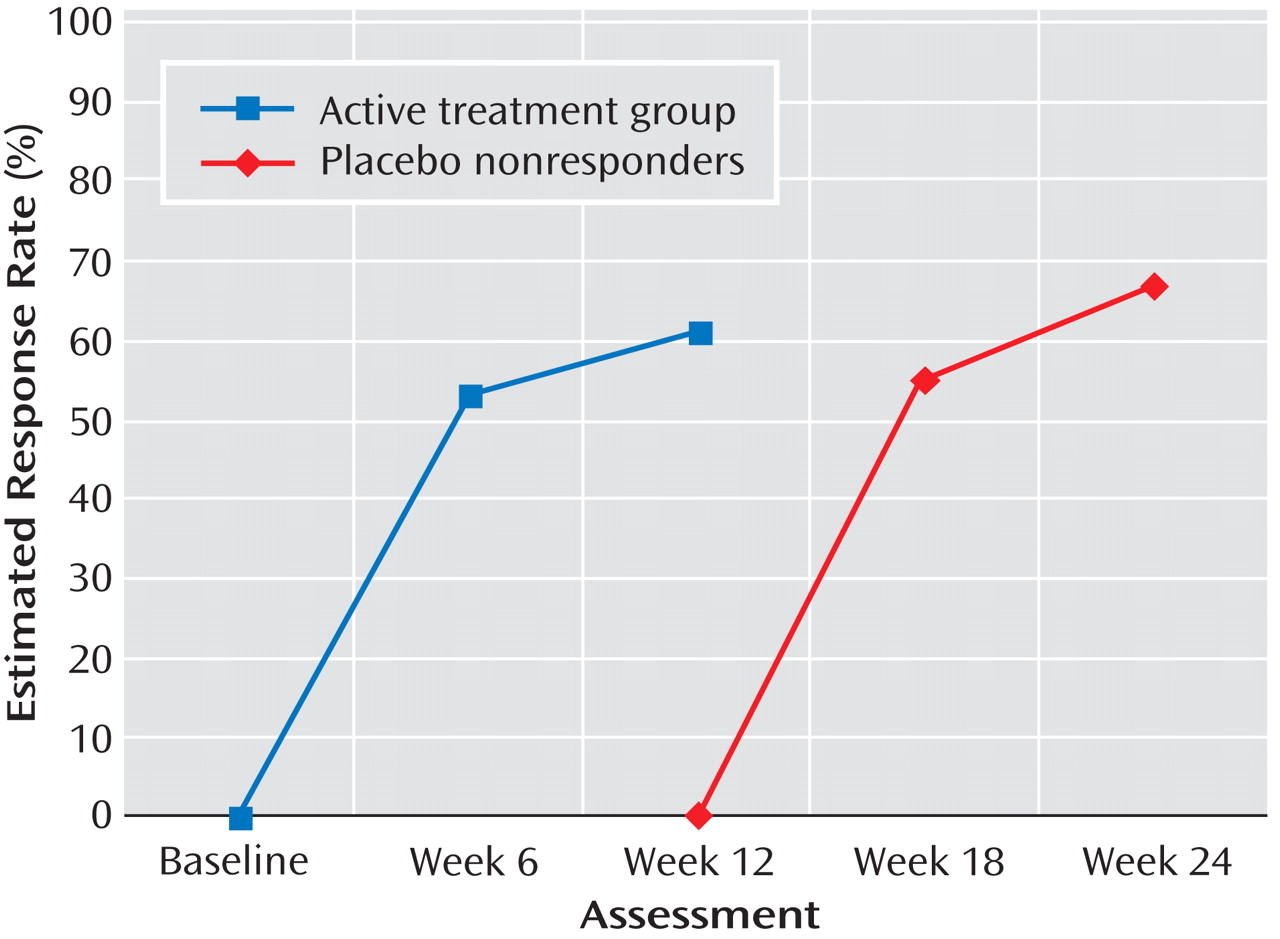
For the placebo/open and active treatment groups, respectively, response rates were 35% and 58% at week 12; 67% and 67% at week 18; 72% and 75% at week 24; 70% and 80% at week 30; and 82% and 83% at week 36. Thus, at week 36, response rates were comparable (odds ratio=1.22, 95% confidence interval [CI]=0.62–2.40, p=0.86). Although the treatment-by-assessment week interaction was nonsignificant, the main effects for treatment (χ 2 =4.10, df=1, p=0.043) and assessment week (χ 2 =72.16, df=4, p<0.001) were significant. Site was not a significant covariate. Given the significant treatment main effect, paired contrasts at each assessment indicated that the active treatment group had a significantly higher response rate at week 12 compared with the placebo/open group (χ 2 =17.83, df=1 p<0.001), with no other assessment points yielding significant differences.
Figure 3 highlights the similarity between the response rates for the active treatment group from baseline to week 12 and rates during weeks 12–24 for patients in the placebo/open group who had not responded by week 12 (N=73).
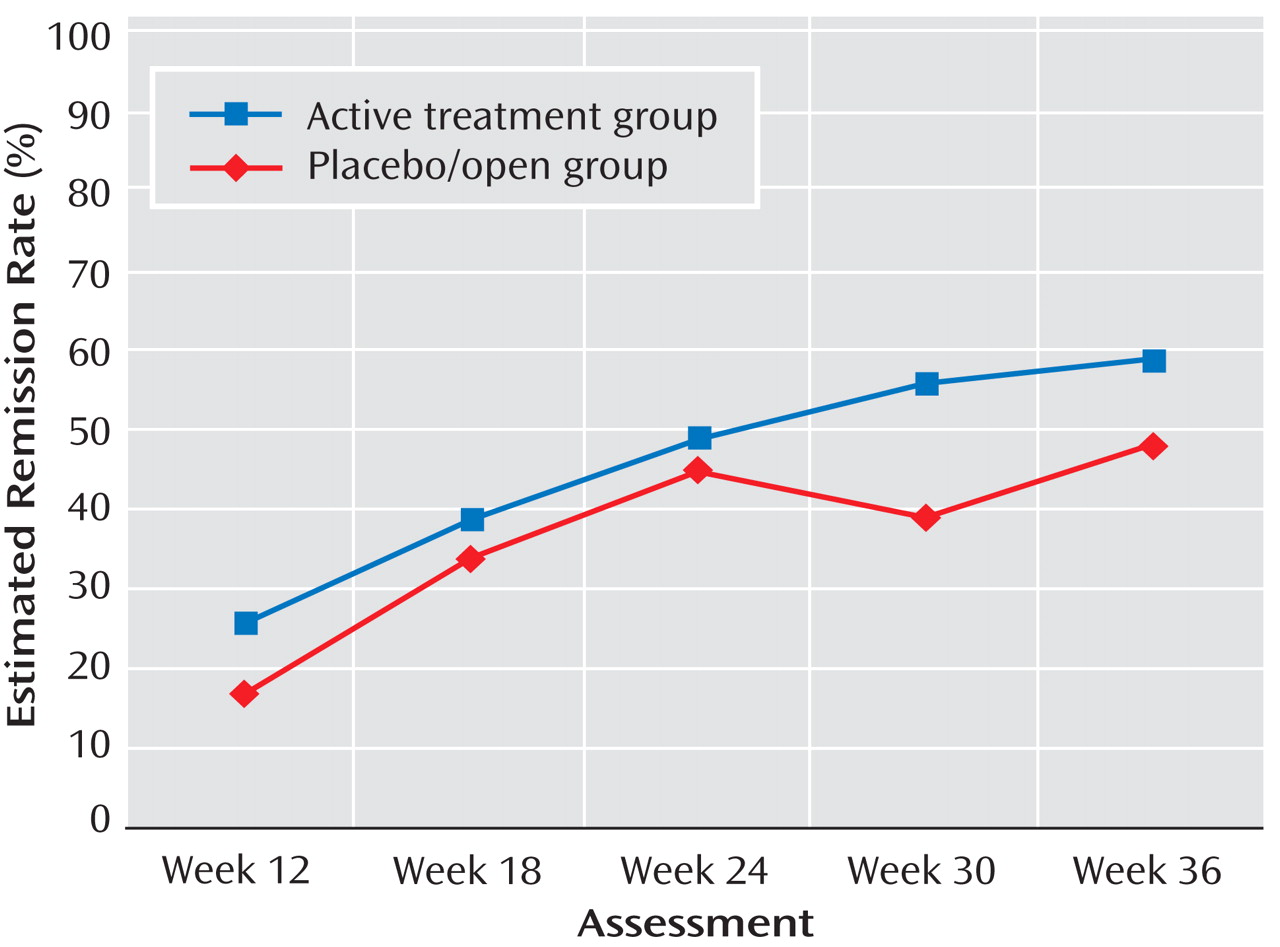
CDRS-R remission rates for placebo/open in stages 2 and 3
The remission rates from weeks 12 through 36 estimated from generalized estimating equation models for the placebo/open and active treatment groups are presented in
Figure 4 . For the placebo/open and active treatment groups, respectively, remission rates were 17% and 26% at week 12; 34% and 39% at week 18; 45% and 49% at week 24; 39% and 56% at week 30; and 48% and 59% at week 36. Again, at week 36, remission rates were similar (odds ratio=1.71, 95% CI=1.01–2.89, p=0.06). The treatment-by-assessment week interaction was nonsignificant, but the main effects for treatment (χ
2 =5.94, df=1, p<0.015) and assessment week (χ
2 =57.63, df=4, p<0.001) were significant. Site was a significant covariate (χ
2 =32.26, df=9, p<0.001), indicating that sites differed in remission rates across time. Paired contrasts indicated that participants in the active treatment group had a significantly higher probability of remission at week 30 compared with those in the placebo/open group (χ
2 =6.74, p<0.010). No other assessment points yielded significant differences between the active treatment and placebo/open conditions.
Estimated remission rates were calculated at week 36 for patients identified as placebo responders at week 12 (last observation carried forward; N=39). Those who responded to placebo at week 12 had an estimated week 36 remission rate of 61%; placebo nonresponders had an estimated rate of 39.7% (χ 2 =3.3, df=1, p=0.07).
Risk of Harm From Withholding Active Treatment
Serious adverse events and primary suicidal events in the placebo/open group
The rate of serious adverse events in the placebo/open group over 36 weeks of treatment was 13.4% (15/112), which was not significantly different from the rate of 11.3% in active treatment group (37/327). Of the 112 participants assigned to the placebo/open group, 12 (10.7%) had a primary suicidal event (a suicide attempt, preparatory action toward suicidal behavior, or suicidal ideation) between week 12 and week 36, compared with 32 of the 327 (9.8%) patients in the active treatment group, a difference that was not statistically significant.
Worsening after acute treatment
The rate of worsening between weeks 12 and 36 for the placebo/open group was 8.0% (9/112), which was not significantly different from the active treatment group rate of 8.6% (28/327).
Rescue Procedures to Minimize Possible Negative Consequences From Placebo Use
The use of rescue/adjunct services to prevent attrition was assessed at weeks 12, 24, and 36. No significant differences were found between groups at week 12, with 17.4% (57/327) of active treatment patients receiving these services, compared with 17.0% (19/112) in the placebo/open group. At week 24, a significantly smaller proportion of the placebo/open group (6.25%; 7/112) required rescue/adjunct visits than the active treatment group (18.4%; 60/327; χ 2 =9.44, p<0.002). At week 36, none of the 112 patients in the placebo/open group required rescue/adjunct visits, compared with 31 of 327 (9.5%) in the active treatment group (χ 2 =11.42, p<0.001).
Comparisons of Placebo and SSRI Treatments
To determine whether the findings described above held without the CBT condition, we constructed a group that included participants who received a selective serotonin reuptake inhibitor (SSRI)—that is, those in the fluoxetine and combination treatment groups. The rationale for constructing an SSRI group was to compare participants in the placebo/open group with those treated with an active antidepressant, eliminating the CBT group, which did not statistically separate from placebo/open at 12 weeks
(7) . On all of the outcomes described above, comparisons between the placebo/open and SSRI groups yielded the same pattern of results as the active treatment group.
Discussion
Our purpose in this study was to evaluate the safety of using placebo as a control in clinical trials involving adolescents with moderate to severe depression. To our knowledge, there have been no published reports of long-term outcomes in adolescents treated with placebo. Our findings indicate that depressed adolescents initially treated with placebo for 12 weeks and subsequently treated as clinically indicated did not have significantly poorer long-term outcomes, despite having a poorer outcome at 12 weeks. Interestingly, the majority of those in the placebo/open group who did not respond during acute treatment (72.6%) elected to receive TADS treatment afterward, which suggests that participants were willing to accept a trial of active treatment after 12 weeks of placebo.
As expected, response rates differed between the placebo/open and active treatment groups at the end of 12 weeks of treatment. However, the response rate among placebo/open participants who went on to receive 12 weeks of active TADS treatment during weeks 12–36 mirrored that of participants who received active treatments in the acute phase of TADS. Thus, delaying active treatment for 12 weeks did not adversely affect participants’ ability to have an adequate treatment response when active treatment was later provided.
Rates of remission, a more conservative outcome measure than response, did not differ at week 12 between placebo/open and active treatment patients. The long-term results at week 36 showed no significant difference in remission rates between placebo/open and active treatments, which suggests that patients whose treatment is delayed are able to achieve nearly the same rates of remission as those who receive active treatment from the start. Remission outcomes differed between placebo/open and active treatment at week 30. It is unclear why remission was lower in the placebo/open group. One possible reason is that placebo/open nonresponders were offered only 12 weeks of open TADS treatment (followed by 12 weeks of uncontrolled continued care), as opposed to the active treatment patients, who received as much as 36 weeks of TADS treatment. Alternatively, it could reflect the delay in receipt of active treatment, although the response rate in this group after 12 weeks of TADS treatment was comparable to that of the active treatment group during their initial 12 weeks of treatment. Notably, those who had responded to placebo by week 12 had a relatively high remission rate at week 36 (61%), indicating that the positive outcomes for this group remained.
Rates of suicidal events and clinical worsening did not differ between patients in the placebo/open group and those in active treatments. In addition, the cumulative dropout rates at week 36 did not differ between groups. This suggests that assignment to placebo during acute treatment does not increase harm-related events, including suicidality, and does not adversely affect long-term retention in a treatment study. It is important to note that the large majority of suicide attempts among placebo/open patients (80%; 12/15) occurred after the period during which they were on the placebo. Thus, depressed adolescents assigned to placebo condition are for the most part able to tolerate the waiting period without significant clinical distress.
Participants initially assigned to placebo had a lower utilization of crisis interventions during weeks 12–36 than those initially assigned to active treatment conditions, even though during weeks 12 to 24, most placebo/open participants were receiving open TADS treatments. The low crisis intervention utilization, combined with there being no difference in rates of suicidal and serious adverse events between the groups, suggests that placebo/open participants are no more in need of rescue procedures than those in active treatment.
At the end of acute treatment, most (73%) placebo/open participants eligible for active treatment elected to receive TADS combination or fluoxetine treatment. It may be that these participants selected a medication treatment because they already had a relationship with the pharmacotherapist and were comfortable with the procedures associated with medication intervention. It is unclear why 27% of placebo/open participants who did not respond during placebo treatment opted not to receive TADS treatment after the acute phase.
Finally, placebo/open participants tended to use more mental health services at week 36. This is likely related to the end of the TADS open treatment after 24 weeks. In addition, placebo/open participants were offered a smaller total dose of care (12 versus 36 weeks for active treatment patients). On a positive note, the higher utilization also can be interpreted as suggesting that these patients were not reluctant to seek additional treatment as a result of participating in the placebo/open condition.
Our results are limited by the definitions used for response and remission. Different definitions would likely yield different findings. In addition, since the focus of this study was on longer-term outcomes, all of the participants were in open treatment, and many of them, including all in placebo/open group, were no longer in their initially randomized condition, which somewhat limits our ability to interpret findings associated with a given treatment condition. Also, the focus on longer outcomes resulted in smaller sample sizes due to attrition.
Conclusions
This purpose of this study was to assess whether placebo is an ethically acceptable control condition in terms of the potential for direct benefit, risk of harm from withholding active treatment, and availability and use of rescue procedures to minimize negative consequences. In all respects, our findings reveal that patients treated with placebo during acute treatment went on to achieve outcomes similar to those of patients who received active interventions, despite having received less treatment overall than those initially treated with an active intervention. Hence, our findings suggest that patients treated with placebo acutely are not harmed and that placebo is an acceptable intervention in randomized controlled trials with adolescents who have moderate to severe depression. While acceptable for a research study, delaying the onset of meaningful treatment in non-research settings is not ethical or clinically appropriate. However, placebo-controlled trials continue to be an important and acceptable research tool for establishing the safety and efficacy of new interventions for depressed adolescents.