Early-onset schizophrenia and schizoaffective disorder occurring prior to 18 years of age are associated with debilitating psychotic symptoms and psychosocial dysfunction
(1,
2) . Prognosis appears to be substantively worse than in adult-onset schizophrenia
(1,
3 –
7) . Safe and effective treatments are needed for these vulnerable youth. Most clinicians prescribe atypical (second-generation) antipsychotics based on assumptions of superior efficacy and tolerability
(8) . However, for adults with schizophrenia, the results of studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)
(9), the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
(10), and the European First Episode Schizophrenia Trial
(11) raise questions as to whether second-generation antipsychotics truly have superior efficacy over first-generation (typical) antipsychotics. Studies specifically comparing second-generation to first-generation antipsychotics for first-episode schizophrenia have had mixed results, with advantages for second-generation antipsychotics often small or limited to secondary outcomes
(12 –
16) .
There are few randomized, controlled trials comparing treatments for early-onset schizophrenia and schizoaffective disorder. First-generation antipsychotics, such as haloperidol and loxapine, have shown efficacy
(17,
18), but younger patients may be at higher risk for extrapyramidal side effects
(19) and less responsive to these agents than adults
(20) . Among second-generation antipsychotics, recent randomized, controlled trials found that olanzapine
(21), risperidone
(22), and aripiprazole
(23,
24) have shown efficacy in the acute treatment of adolescents with schizophrenia. Risperidone and aripiprazole have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of adolescents with schizophrenia. Clozapine, a second-generation antipsychotic, was found to be superior to both haloperidol
(25) and olanzapine
(26) in youth with treatment-resistant schizophrenia. However, clozapine’s side effect profile
(27) limits its use to patients who have tried and failed other antipsychotics.
The publicly funded study Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) was designed to rigorously compare the efficacy and safety of a first-generation antipsychotic, molindone, with two second-generation antipsychotics, olanzapine and risperidone, in the treatment of early-onset schizophrenia and schizoaffective disorder. The primary hypothesis was that treatment with olanzapine and risperidone would be associated with greater treatment response and greater tolerability than treatment with molindone. The safety information gained may also inform the widespread use of second-generation antipsychotics for pediatric behavioral and mood disorders.
Method
Study Setting and Design
The rationale and design of TEOSS have been detailed previously
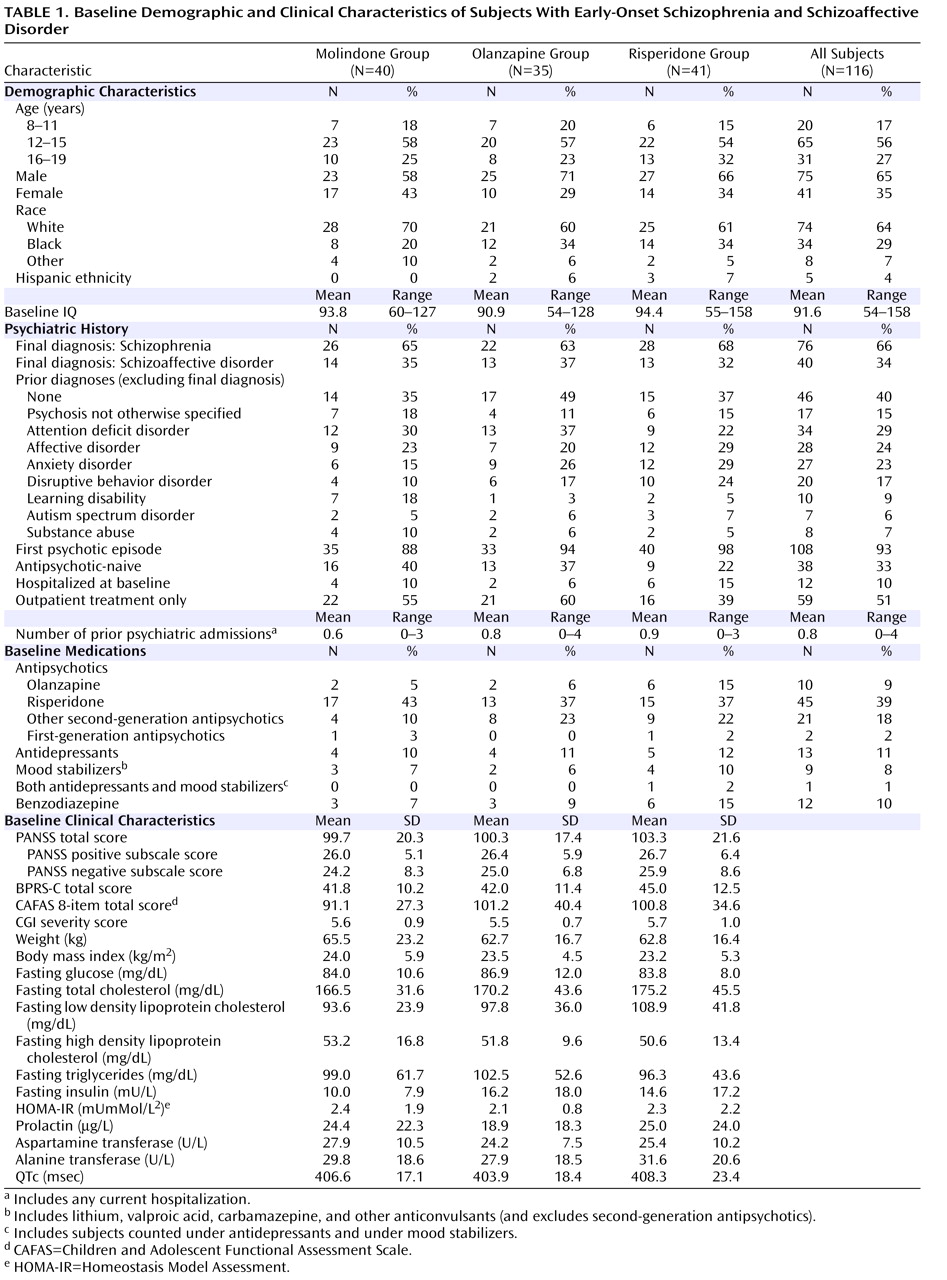
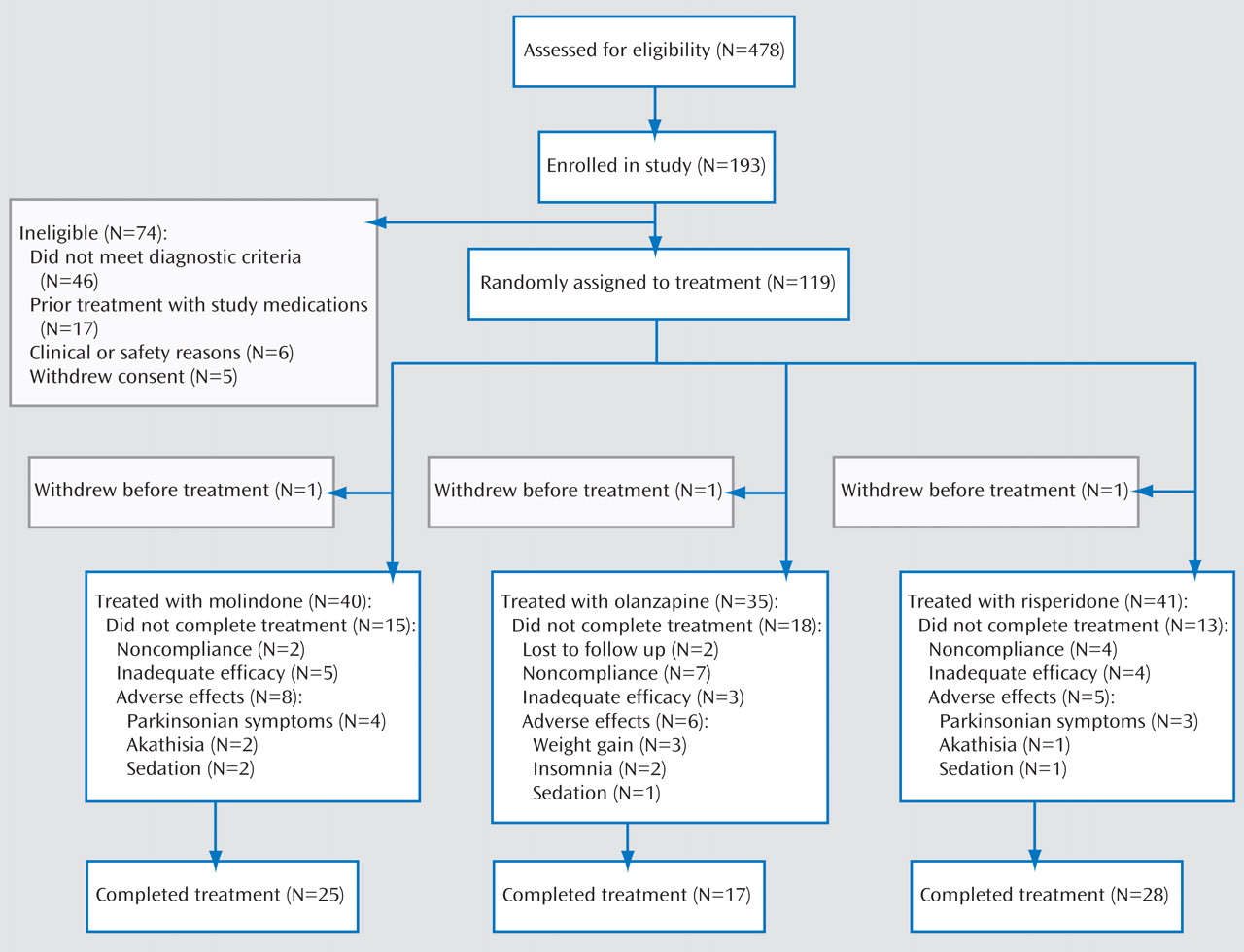
(28) . Eligible subjects were randomly assigned to either molindone, olanzapine, or risperidone treatment under double-blind conditions for 8 weeks. The study was designed to have a total of 168 subjects equally distributed among three groups (N=56 in each treatment group) and to have 80% power to detect differences in response rates of 45% (molindone), 60% (risperidone), and 75% (olanzapine). The sample size available for analysis was 116 subjects, limiting our power to detect between-group differences less than 18%.
From February 2002 to May 2006, youth were screened at four academic sites: University of North Carolina at Chapel Hill, McLean Hospital and Cambridge Health Alliance at Harvard Medical School, University of Washington, and Case Western Reserve University. The study was reviewed and approved by the institutional review board at each site. Participant safety was also monitored regularly throughout the study by the National Institute of Mental Health (NIMH) Data and Safety Monitoring Board.
Participants
Eligible participants were 8–19 years old, with a focus on primarily younger participants, so that 30% or fewer of subjects were between 16 and 19 years old. Participants had a diagnosis of schizophrenia, schizoaffective disorder, or schizophreniform disorder and had current positive psychotic symptoms of at least moderate intensity, as rated on the Positive and Negative Syndrome Scale (PANSS)
(29) . DSM-IV diagnoses were made by a child psychiatrist and confirmed with the Children’s Structured Clinical Interview for DSM-IV (KID-SCID) (30) and a teleconference among the principal investigators. The KID-SCID uses the same mood, psychosis, and substance abuse modules as the Structured Clinical Interview for DSM-IV (SCID)
(31) . It was used to provide continuity with adult studies, which frequently use the SCID, and because its psychosis module has been used in studies of the longitudinal course of early-onset schizophrenia and schizoaffective disorder
(6,
32) . The initial diagnosis was reviewed upon completion of the study and revised as indicated, including reclassifying all those initially diagnosed with schizophreniform disorder. Individuals with prior evidence of mental retardation; current major depressive episode; active substance abuse; history of intolerance or nonresponse to any of the study treatments during a prior episode; history of an adequate trial of any of the study treatments during the current episode (defined as at least 8 weeks of treatment, including at least 2 weeks at the maximal dose allowed in the current study), or those individuals felt to be at imminent risk of harming themselves or others were excluded from the study. All participants with prior exposure to one of the study medications had the opportunity for greater drug exposure during the trial. All participants and their guardians provided written informed consent.
Interventions
Study medications were packaged in identical color-coded capsules. Dosing was flexible, allowing for clinician judgment within the following dose ranges: molindone, 10–140 mg/day; olanzapine, 2.5–20 mg/day; and risperidone, 0.5–6 mg/day. Medications were initiated at the lowest dose within the range and typically increased to the middle of the dose range within 10 days for those subjects age 12 years and older and within 14 days for those ages 8–11 years, according to the age-specific schedules provided elsewhere
(28) . All participants treated with molindone also received 1.0 mg benztropine; all others received a placebo identical in appearance.
Antipsychotics and side effect medications in use at the time of random assignment were cross-tapered during the first 2 weeks of study treatment. Individuals whose mood symptoms had been well controlled on a stable dose of antidepressants or nonantipsychotic mood stabilizers for at least 4 weeks prior to study entry were allowed to continue those treatments during the study. Concomitant treatments with anticholinergic agents, propranolol, and benzodiazepines were guided by algorithms
(28) .
Outcomes
The primary outcome was responder status at the end of the acute trial. Responder status was defined a priori as a Clinical Global Impression (CGI)
(33) improvement score of 1 (“very much improved”) or 2 (“much improved”), plus ≥20% reduction in baseline PANSS score and the ability to tolerate 8 weeks of treatment. Individuals who withdrew prior to 8 weeks were considered nonresponders.
Additional efficacy measures included the PANSS positive and negative symptom subscales (an assessment of schizophrenia symptoms widely used in adults), the Brief Psychiatric Rating Scale for Children (BPRS-C)
(34), and the Child and Adolescent Functional Assessment Scale
(35) . In each of these measures, higher scores reflect more severe symptoms. A combination of adult measures and child measures was used to fully assess psychotic symptoms and to establish validity of adult measures in this population.
Secondary safety and tolerability outcomes included neurological side effects, changes in weight and stature, vital signs, laboratory analyses, ECG analyses, and incidence of systematically elicited adverse events
(36), serious adverse events, and treatment discontinuation for any reason. The Simpson-Angus Rating Scale
(37), Barnes Rating Scale for Drug-Induced Akathisia
(38), and Abnormal Involuntary Movement Scale (AIMS)
(18) were employed to monitor extrapyramidal symptoms. Fasting metabolic parameters, prolactin levels, and routine blood and urine chemistries were monitored at weeks 0, 4, and 8. All other outcome measures, except the Child and Adolescent Functional Assessment Scale, were assessed weekly.
Diagnostic and outcome assessments were performed by clinicians blind to study treatment with experience in working with psychotic youth; these clinicians established and maintained interrater reliability on the KID-SCID, CGI, PANSS, and BPRS-C, with an intraclass correlation of ≥0.80 at in-person, cross-site meetings at the beginning and midpoint of the trial and within each site every 6 months. Reliability on the KID-SCID and CGI scale was assessed using vignettes; reliability on the PANSS and BPRS-C was assessed using taped interviews.
Statistical Analyses
After 90 participants had completed the acute trial, an interim analysis of responder status and key safety variables was made available to NIMH’s Data and Safety Monitoring Board, but not other study personnel. Participants with at least one assessment after taking study medication comprised the intent-to-treat analysis population. In both the interim and final analyses, a Mantel-Haenszel chi-square test was used for the primary analysis of responder status. A three-way test was used rather than three separate pairwise comparisons to maximize statistical power, given the limited sample size. Statistical analyses for continuous secondary endpoints used last observation carried forward paired t tests to explore within-treatment effects and one-way analysis of variance (ANOVA) on last observation carried forward endpoints to compare groups. As a sensitivity analysis, we used a mixed model approach to repeated measures for the PANSS total score. Each distinct inference domain (e.g., symptoms, neurological effects, weight, and laboratory analyses) was analyzed independently. Consideration of multiple comparisons should be made within the given inference domain, such that significance should be assumed only if p<0.05/number of assessments in that domain. Metabolic analyses included only those laboratory results obtained while youth were fasting. Differences in the ability to sustain treatment were examined using Kaplan-Meier survival curves. All analyses included site as a covariate. Proportions of each group experiencing specific adverse events were compared with chi-square tests. To avoid overlooking potentially important adverse events, no corrections for multiple comparisons were made.
Discussion
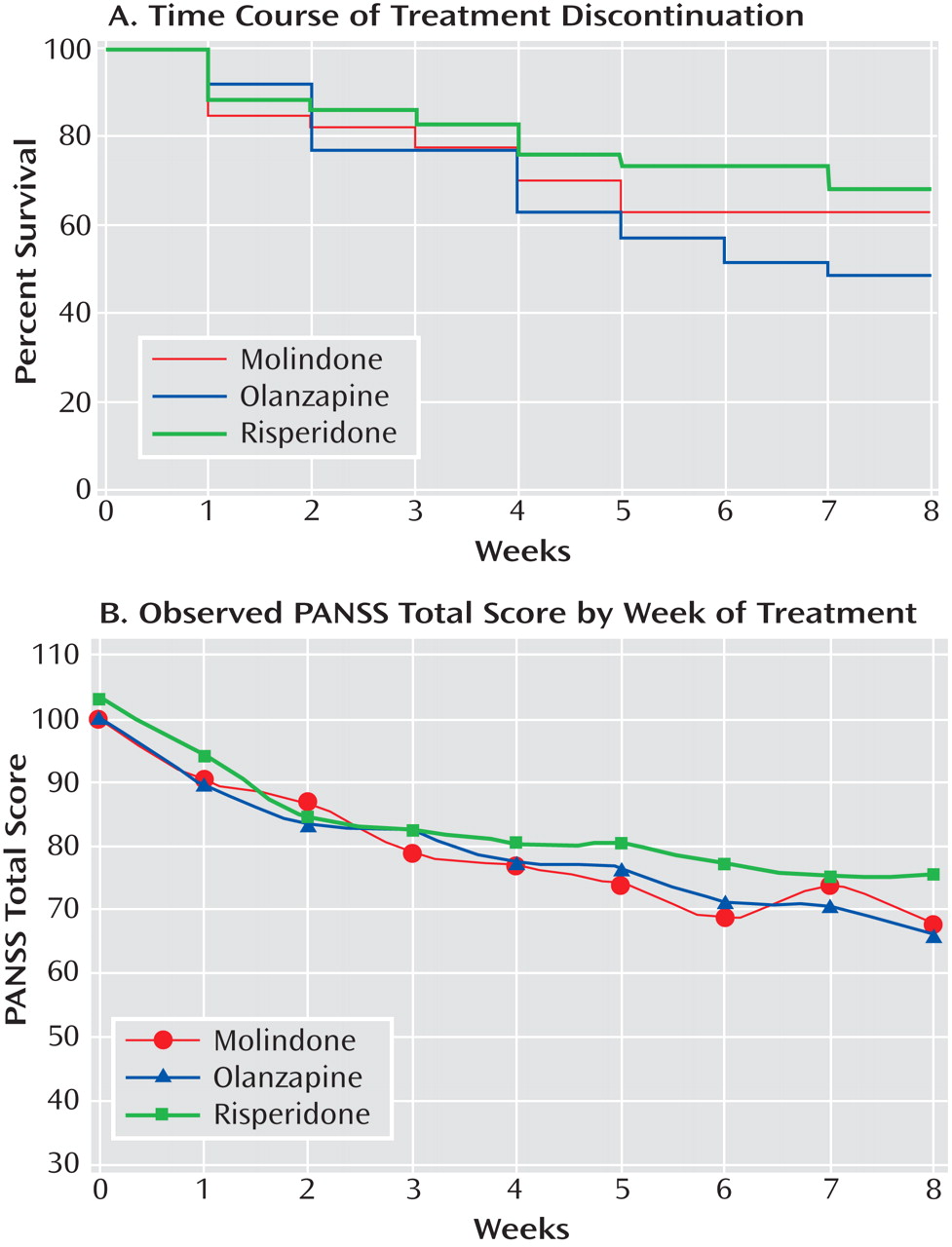
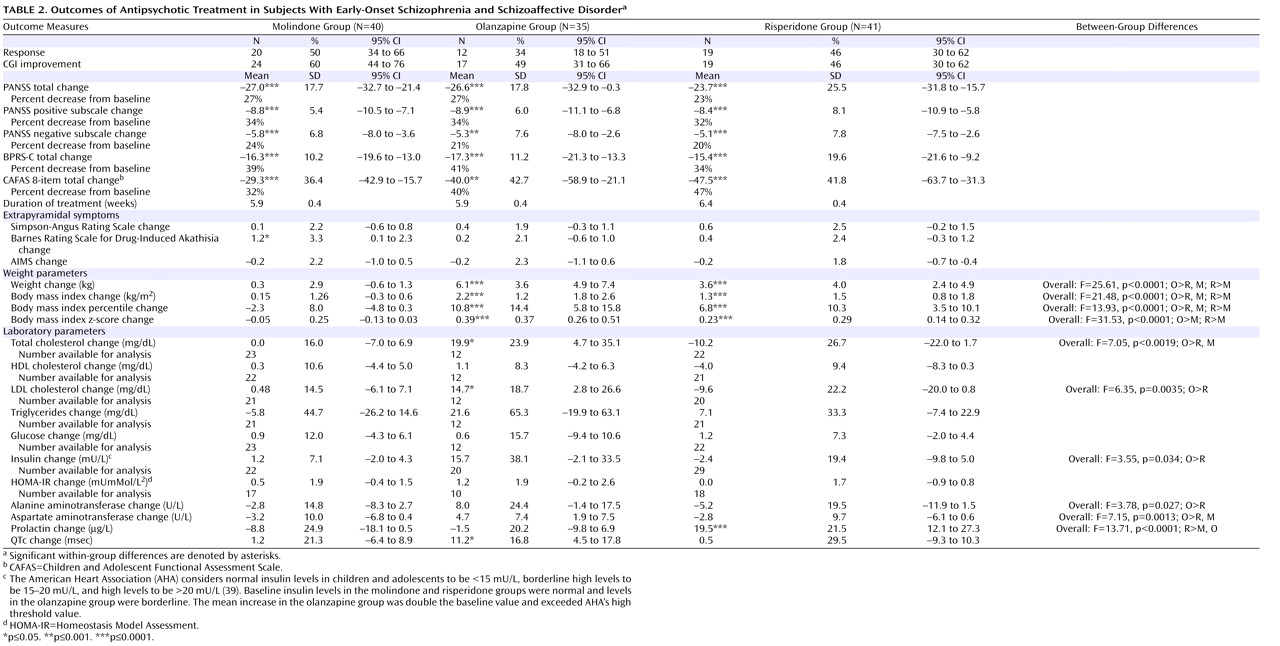
Second-generation antipsychotics olanzapine and risperidone did not demonstrate superior efficacy to molindone in the treatment of children and adolescents with early-onset schizophrenia and schizoaffective disorder. Across all three treatments, more than half the participants failed to achieve an adequate response after 8 weeks of therapy. The response rates were generally lower than those reported in studies of young adults with first-episode schizophrenia using similar criteria
(13,
16,
40,
41) . The response rate was similar to what has been reported in three recent studies of olanzapine in early-onset schizophrenia and schizoaffective disorder lasting 8, 12, and 6 weeks, respectively
(26,
42,
43), but lower than what has been reported to the FDA for a 6-week trial of risperidone
(22) . The mean reductions in psychotic symptoms were modest, ranging from 20%–34% on the PANSS and 34%–41% on the BPRS-C. Furthermore, 10 participants (8%) required hospitalization during the acute trial, primarily as a result of increased psychotic symptoms. Doses of all medications were in the middle of the permitted ranges and generally considered moderate doses. Olanzapine and risperidone mean doses were comparable to those reported to the FDA for pediatric exclusivity studies and in studies of first-episode schizophrenia, but olanzapine doses were lower than those used in treatment-resistant samples
(26,
42) or in chronic samples, such as those used in the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and CATIE.
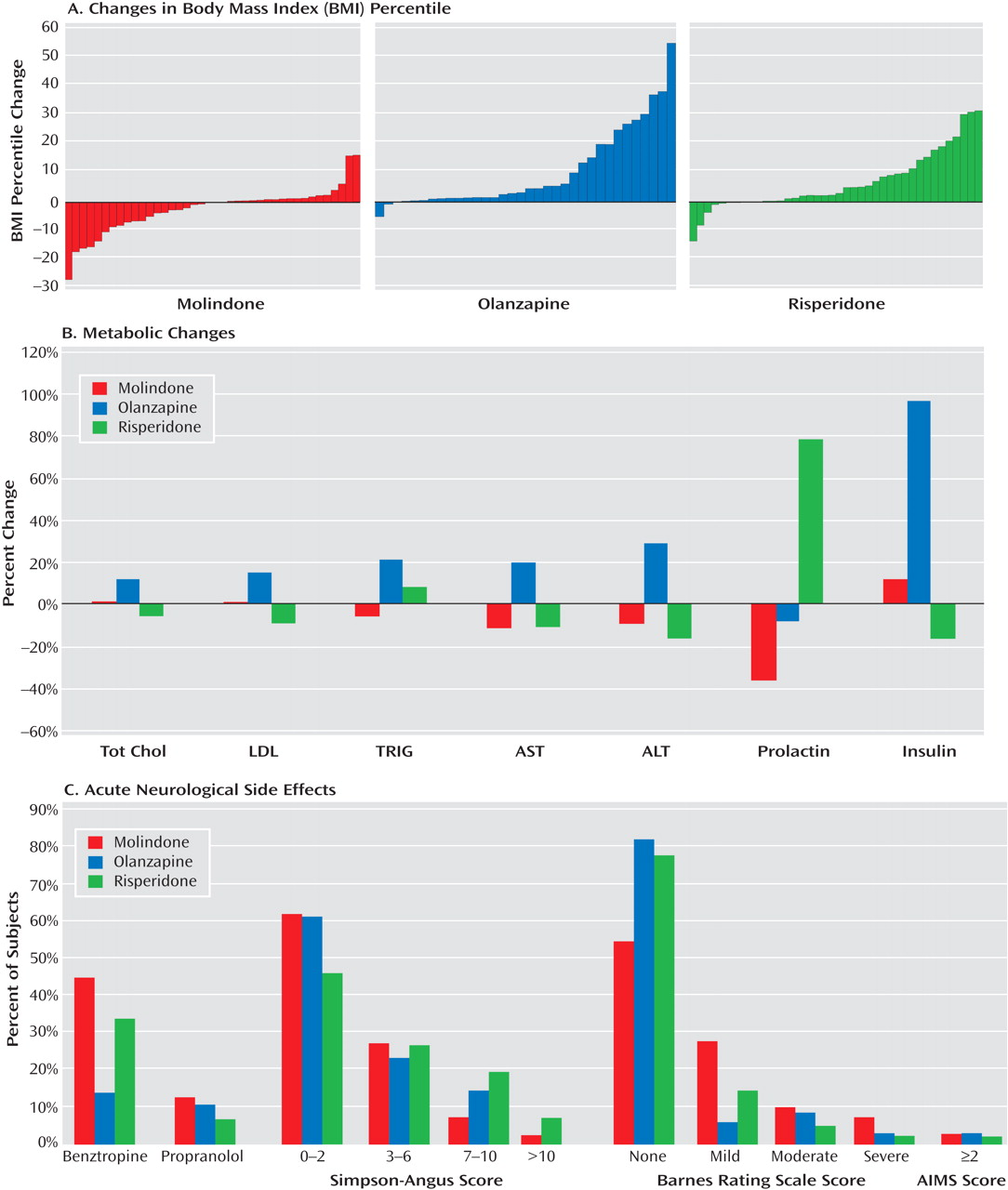
The three treatments did have significantly different safety profiles. Olanzapine resulted in more weight gain than either of the other medications and was uniquely associated with increases in lipid and insulin levels and liver function tests. Clinically significant changes in fasting glucose levels were not observed over the 8 weeks of treatment. This is not surprising, given that youth have large insulin reserves. The risks of obesity, dyslipidemia, and hyperinsulinemia associated with acute treatment generate considerable long-term risks for diabetes and cardiovascular disease. Prolactin levels were uniquely elevated with risperidone treatment, although the long-term health consequences of this are unclear. Subjects receiving molindone did report the adverse effect of akathisia more than those receiving second-generation antipsychotics. However, molindone treatment was not associated with more parkinsonian or dystonic symptoms than olanzapine or risperidone, likely due to prophylactic benztropine treatment. Although it is difficult to rank the clinical importance of different adverse effects, those associated with olanzapine and risperidone are likely to have persistent effects on long-term physical health, while those associated with molindone seem more likely to impact adherence to antipsychotic medication. However, in this trial, there was no greater attrition in the molindone group, despite more reports of akathisia.
The sample included youth with both first-episode and chronic early-onset schizophrenia and schizoaffective disorder, both treatment-naive and antipsychotic-exposed individuals, and some individuals taking concomitant mood disorder medications. Although very few of the patients were hospitalized at the time of enrollment, many had been hospitalized previously
(2) . However, the sample did fully reflect the racial and ethnic populations of the study sites. African American subjects were overrepresented (29% rather than the expected 15%), and Hispanic subjects may have been underrepresented (4% rather than the expected 6%–7%). This may reflect economic biases among Caucasian and African American individuals regarding willingness to participate in clinical research, as well as some cultural or language barriers in the Hispanic community. Nonetheless, the results should generalize well to community settings. The overall low rates of response have significant clinical and public health implications.
This study may also inform the design of future treatment studies in early-onset schizophrenia and schizoaffective disorder. Such studies will likely need to involve a large number of sites with outpatient services to recruit a sufficient number of participants. Furthermore, with the increasing use of antipsychotics for multiple indications, it will likely be difficult to recruit a large proportion of antipsychotic-naive patients. This may be particularly true for younger patients, who are more likely to have significant behavioral problems prior to onset of clear psychotic symptoms. This may suggest the need for establishing drug-free baselines, although the ethical considerations and potential impact on recruitment would need to be carefully considered. It also may be important to conduct trials of longer duration to determine the prevalence of slow response to treatment in this pediatric population, although again, the ethical ramifications of this would need to be considered.
Limitations
The most significant weakness of this study was the sample size, which was sufficient only to detect large differences across the three treatments and limited our ability to identify predictors of response or adverse effects. Although the study was designed to have sufficient power to detect moderate treatment effects, we were not able to achieve the designed sample size. Furthermore, only small differences among treatments emerged, which was not what we predicted. We did not find any evidence of superiority of the two second-generation antipsychotics, risperidone and olanzapine, over the first-generation antipsychotic, molindone. Since current community standards generally consider second-generation antipsychotics to be the first-line treatment for early-onset schizophrenia and schizoaffective disorder, the failure to demonstrate superiority of risperidone and olanzapine is an important finding.
The decision to provide prophylactic benztropine to all youth treated with molindone may have minimized differences in extrapyramidal symptoms among the medications. In addition, anticholinergics like benztropine may have significant adverse neurocognitive effects. However, the administration of prophylactic benztropine with first-generation antipsychotics is common practice. The duration of treatment in the study did not provide information about side effects that appear later, such as tardive dyskinesia.
Finally, different choices could have been made with regard to the specific medications studied. At the time the trial was initiated, olanzapine was widely used in the pediatric population, whereas quetiapine had a small market share. Ziprasidone and aripiprazole, both of which may have fewer metabolic side effects, were introduced subsequent to the initiation of the study. Efforts to introduce them partway through the study were not supported by the FDA or NIMH. We also considered utilizing a placebo for comparison, as opposed to a first-generation antipsychotic. We expected that this would increase the demonstrated efficacy of the second-generation antipsychotics, but it would not address the fundamental comparative questions. Distributing the sample among four treatment conditions rather than three would also have reduced statistical power. We also considered requiring a drug-free baseline to minimize the likelihood of finding no apparent benefit of substituting one partially effective treatment for another. However, concerns about the long-term consequences of delaying effective treatment and associated recruitment difficulties argued against including a placebo treatment group or a drug-free baseline. At the time the study was initiated, there were significant ethical concerns about utilizing any first-generation antipsychotic in comparison with second-generation antipsychotics, because second-generation antipsychotic treatment was the standard of care for early-onset schizophrenia and schizoaffective disorder. We felt any traditional medication selected as a comparator would have to provide a strong potential advantage to maintain therapeutic equipoise. Molindone was chosen as the best option among first-generation antipsychotics based on its low propensity for both weight gain and extrapyramidal side effects. Despite this advantage, molindone is not commonly used in clinical practice. A more frequently used medication, such as perphenazine or haloperidol, might have facilitated comparison with adult studies and acceptance in the community. Failure to require a drug-free baseline may have reduced response rates and led to earlier treatment discontinuation.
Another potential limitation of the study is the 8-week duration of treatment. Different patterns of response or risk of side effects might have emerged over a longer trial. Some young people may require more extended therapy to adequately respond, and it is likely that some aspects of the illness, such as negative symptoms, neurocognitive function, and associated anxiety, may require longer periods to recover
(44,
45) . However, published standards of care for early-onset schizophrenia and schizoaffective disorder recommend the use of 6- to 8-week trials
(1) . A longer acute phase trial would have increased the risk of exposing subjects to prolonged ineffective treatment. Furthermore, antipsychotic medication trials in adults with schizophrenia suggest that nonresponse as early as 2–4 weeks after initiating treatment predicts nonresponse up to 12 weeks later
(46 –
49) .
Conclusions
The results of this study do not support the widely held assumption that risperidone and olanzapine, two of the most widely used second-generation antipsychotics, are superior to an advantageous first-generation antipsychotic for the treatment of early-onset schizophrenia and schizoaffective disorder. The safety data underscore the risks of weight gain and metabolic side effects with some second-generation antipsychotics, particularly olanzapine, and the importance of closely monitoring weight, glucose and lipid levels, and liver functioning.
These findings have broad public health implications. In the long term, the metabolic side effects of olanzapine and risperidone may place many youth at risk for diabetes and cardiovascular problems. Second-generation antipsychotics are now widely used to treat nonpsychotic mood and behavioral disorders in youth. The balance between potential therapeutic benefits and risk of adverse events needs to be carefully considered in this age group.
Further analyses from the Treatment of Early-Onset Schizophrenia Spectrum Disorders study will examine the outcomes and adverse effects of these treatments over 1 year. Additional studies with larger sample sizes are critically needed to compare the efficacy and side effect profiles of other widely used second-generation antipsychotics and midpotency first-generation antipsychotics in youth. Strategies to reduce weight gain and metabolic impact of antipsychotics must be developed and evaluated. The limited response in this study to all three medications, with fewer than 50% of subjects completing 8 weeks of treatment, emphasizes the need to develop more effective treatments for early-onset schizophrenia and schizoaffective disorder.