Impaired cognitive function has been identified as a core feature of schizophrenia (
1). Functional MRI studies have consistently provided evidence of disturbed cognitive function in patients with schizophrenia relative to comparison subjects (
2–
4), but the pathological processes underlying such impaired cognitive function are not known. Disruptions of white matter networks have been proposed as a potential mechanism for cognitive dysfunction in schizophrenia and in other neurological pathologies such as dementia (
5), multiple sclerosis (
6) and traumatic brain injury (
7).
However, not all patients with schizophrenia show a cognitive impairment. Within the diagnostic category of schizophrenia in DSM-IV there may be a wide range in cognitive performance, from relatively intact cognition to severe deficits. Whether this corresponds to different pathophysiological processes is unclear. In a recent study, Wexler et al. (
8) found that relative to healthy comparison subjects, neuropsychologically impaired patients had significantly smaller white matter volumes in several regions, whereas patients with no cognitive deficit (within 0.5 SD of healthy comparison subjects) did not differ from healthy subjects. The authors suggest that white matter pathology may play a primary role in the cognitive deficits, and that the differences observed between the two subgroups might correspond to differences in the disease process.
Diffusion tensor imaging has been proven to be a useful technique for detecting structural abnormalities in the white matter of individuals with cognitive impairment (
9–
13). In schizophrenia, only a few studies have investigated the association between white matter connections and cognitive performance (
14–
20). The findings to date have been inconclusive, perhaps due to the wide heterogeneity in the study populations and in the imaging methodologies used.
In the present study, using a voxel-based analysis method, we investigated white matter integrity—in patients with and without cognitive deficits—in four domains known to be impaired in schizophrenia: executive function, verbal memory, attention, and motor dexterity. We hypothesized that patients with cognitive impairments would have significantly lower fractional anisotropy than patients without cognitive deficits. More specifically, we predicted that 1) patients with impaired executive functioning would have lower fractional anisotropy in white matter areas containing tracts connecting the prefrontal cortex to temporal and parietal cortices; 2) patients with impaired verbal memory would show lower fractional anisotropy in fronto-temporal pathways (particularly the uncinate fasiculus); 3) patients with impaired attention would demonstrate reduced fractional anisotropy in frontostriatal tracts; and 4) patients with impaired motor functioning would have lower fractional anisotropy in white matter comprising fasciculi linking the inferior frontal and anterior cingulate cortex with subcortical nuclei. To minimize the potential effects of chronic illness and medication exposure, we conducted the study in minimally treated first-episode psychotic patients.
Method
Subjects
Patients were recruited from a consecutive sample of subjects included in the first-episode psychosis program of Cantabria (PAFIP) (
21), Spain. Patients referred to the program were selected if they met the following criteria: 1) age 15–60 years, 2) living in the catchment area, 3) experiencing their first episode of psychosis, 4) no prior treatment with antipsychotic medication or, if previously treated, a total lifetime exposure less than 6 weeks, and 5) meeting DSM-IV criteria for brief psychotic disorder, schizophreniform disorder, schizophrenia, or schizoaffective disorder. Patients were excluded for any of the following reasons: 1) meeting DSM-IV criteria for drug dependence, 2) meeting DSM-IV criteria for mental retardation, or 3) having a history of neurological disease or head injury. Diagnosis per DSM-IV criteria was confirmed by an experienced psychiatrist 6 months after the initial contact. After the patients provided written informed consent, they were randomly assigned to receive aripiprazole (10–30 mg/day), quetiapine (200–600 mg/day), or ziprasidone (40–160 mg/day).
All the patients included in the first-episode psychosis program from February 2006 to June 2008 were invited to participate in the diffusion tensor imaging study. The protocol was approved by the Marques de Valdecilla University Hospital review board and was performed in accordance with international ethical standards. The scans were conducted as soon as the patients could tolerate the procedure following the initiation of treatment. The average time between initiation of medication and MRI scanning was 35.5 days (SD=30, maximum=161 days); the median was 25.5 days.
Healthy comparison subjects were recruited from the community through advertisements. They had no past or present psychiatric, neurological, or general medical illness, including substance abuse or significant loss of consciousness, as determined by using an abbreviated version of the Comprehensive Assessment of Symptoms and History (
22). Healthy subjects were matched to the patients by age, sex, years of education, and laterality index. All participants provided informed consent after the study procedures were fully explained to them.
Cognitive Tasks
Cognitive functioning was evaluated 12 weeks after recruitment. To minimize type I error from our neurocognitive battery (
23), we selected the three tasks with the worst patient performance compared with healthy volunteers (highest mean z scores) along with an additional task to evaluate verbal memory. The tasks were 1) the degraded stimulus portion of the Continuous Performance Test to measure attention; 2) time for dominant hand portion of the Grooved Pegboard Test to evaluate motor dexterity; 3) long delay recall from the Rey Auditory Verbal Learning Test to measure verbal memory; and d) total time on the Trail Making Test Part B to assess executive functioning.
Statistical Analysis
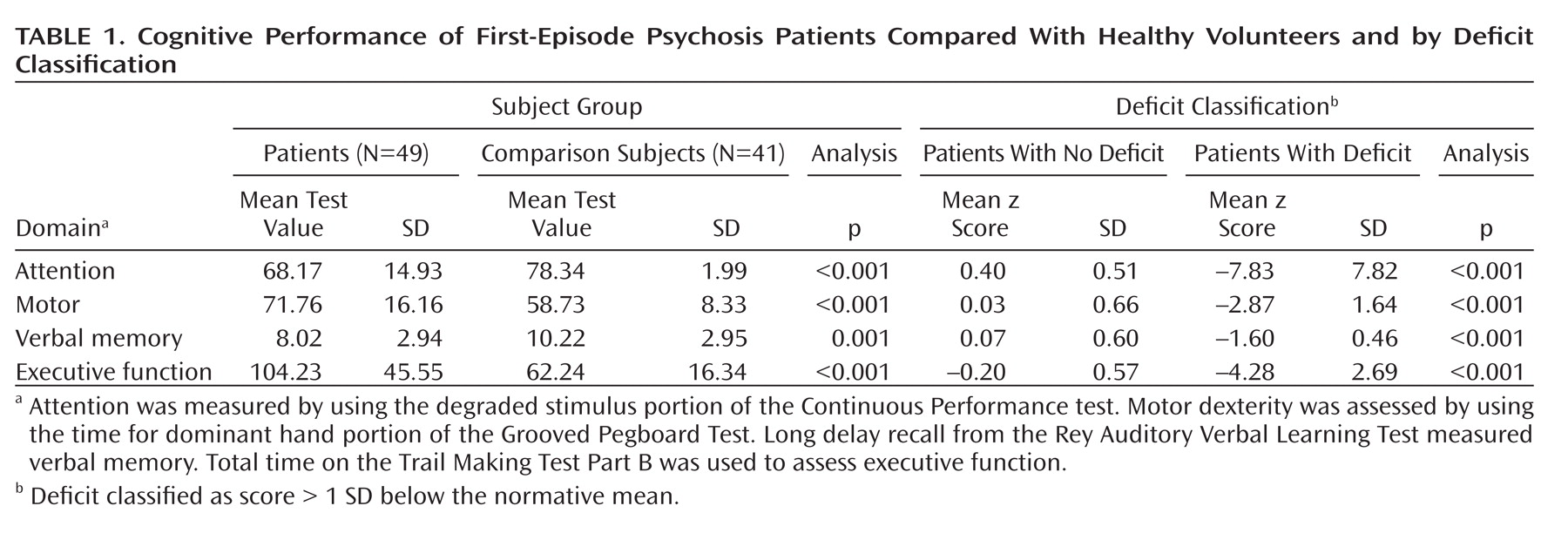
Each raw cognitive score was standardized to a z score (with a mean of 0 and a SD of 1) using the values of the healthy comparison group as reference. Patients were then classified as impaired on each cognitive function if performance was more than 1 SD below the normative mean. Pearson's chi-square for categorical data and Student's t tests for continuous variables were used to compare demographic data between cognitively impaired and unimpaired groups.
Image Acquisition
All MRI scans were obtained at Marques de Valdecilla University Hospital using a 1.5T General Electric SIGNA System (GE Medical Systems, Milwaukee). Diffusion-weighted imaging data were acquired with a single-shot echo planar imaging sequence aligned with the anterior-posterior commissure plane. Images were collected with diffusion sensitizing gradients applied along 25 nonparallel directions (b=1000 s/mm2), along with one image without diffusion weighting (b=0). Twenty-seven contiguous axial slices were acquired with a slice thickness of 5 mm and no gap. The acquisition parameters were as follows: echo time=91 msec; repetition time=8000 msec; field of view=30 cm; number of excitations=1; matrix size=128×128.
Additionally, high resolution structural images were acquired for anatomical review. Three-dimensional T1 weighted images, using a spoiled gradient echo sequence, were obtained in the coronal plane with the following parameters: echo time=5 msec; repetition time=24 msec; number of excitations=2; excitation flip angle=45º; field of view=26×19.5 cm; slice thickness=1.5 cm; and matrix size=256×192. Two-dimensional T2 and proton density weighted sequences were acquired as follows: 3.0-mm-thick coronal slices; repetition time=3000 msec; echo time=36 msec (for proton density) and 96 msec (for T2); number of excitations=1; field of view=26×26; and matrix=256×192.
The total scan time was 40 minutes. All scans were reviewed, and any scan with significant artifacts was repeated or discarded.
Image Processing
The diffusion-weighted images were first corrected for any eddy current-induced distortion and then masked with a modification of the brain extraction tool (BET) in the Functional Software Library (FSL) package (Oxford University, Centre for Functional MRI of the Brain, Oxford, U.K.; see Jones et al. [
24]). The diffusion tensor was then calculated at each brain voxel and fractional anisotropy maps were constructed using in-house software (
25).
A voxel-based method similar to voxel-based morphometry was used to analyze the diffusion tensor imaging images. Registration was performed using SPM2 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, U.K.). A two-stage registration was used, in a manner similar to an "optimized voxel-based morphometry" analysis (
26). First, the mean T
2 weighted (non-diffusion-weighted, b=0) images from each subject were registered to the standard echo-planar imaging template provided by SPM2. The derived mapping parameters for each subject were applied to the coregistered fractional anisotropy images. The normalized fractional anisotropy images of all subjects were then averaged and smoothed to create a new, study-specific template. Finally, the fractional anisotropy images were reregistered to the study template.
The registered fractional anisotropy images were also segmented using SPM's default a priori tissue probability information. These segmented images were thresholded at a value of 0.10 to provide a binary mask of white matter that included all white matter voxels and excluded the voxels that were clearly gray matter or CSF. A 4-mm full-width at half-maximum smoothing filter was used to improve signal-to-noise ratio. The aforementioned mask was used for the smoothed images to restrict subsequent analyses to white matter.
Image Analysis
Between-group comparisons were performed using X-Brain Activation Mapping (XBAM), a program developed at the Institute of Psychiatry, London (
27). XBAM uses nonparametric, permutation-based statistics, which are more suitable for this type of data than parametric approaches, since the residuals of fit to the general linear model may not show the Gaussian behavior required by parametric statistics.
To assess differences between groups, an analysis of variance (ANOVA) was conducted at each intracerebral voxel in standard space. Initially, a liberal statistical threshold (p≤0.05) was set to detect voxels putatively demonstrating differences between groups. Only those voxels at which all subjects contributed data were considered further, which, along with the aforementioned masking procedure, restricts the analysis to core white matter regions, reducing the search volume and thus the number of comparisons.
The program then searches for clusters of contiguous (10-connected) significant voxels in the observed data. For each cluster, it calculates the sum of all suprathreshold voxel statistics within that cluster—termed the cluster "mass." To assess the statistical significance at the cluster level, rather than set a single a priori threshold, we calculated, for a range of p values, the number of clusters that would be expected by chance alone. We then set the statistical threshold for cluster significance for this analysis at a p value such that the expected number of false positive clusters by chance alone would be less than one.
Four separate analyses were conducted, with fractional anisotropy values being compared between patients with and without deficit in each cognitive domain. The four comparisons conducted are all reported at a cluster-level significance threshold of p≤0.0025.
Results
Forty-nine right-handed first-episode psychotic patients and 41 healthy comparison subjects were included in the analyses. Healthy subjects were matched to the overall patient group for laterality, age, sex, and years of education. The findings from the direct diffusion tensor voxel-based morphometry comparison have been reported elsewhere (
28).
Table 1 shows the mean raw scores on the cognitive tasks for patients relative to the comparison subjects. For each cognitive domain, the patient group was divided into two subgroups according to the adjusted z scores. The mean z scores of deficit and nondeficit cognitive subtypes are also shown in Table 1. No significant differences were found in age (the most potential confounding factor for which there is some evidence of influence on fractional anisotropy values), sex, or duration of untreated psychosis between patients with and without deficit in any of the four cognitive domains (see table S1 in the data supplement that accompanies the online version of this article). A difference (at the upper limit for statistical significance [p=0.050]) was found in years of education between the patients showing deficits on the executive function task and those showing no deficits. A significant difference was also found in years of education between the patients with deficit and no deficit in the motor domain (p=0.027). To our knowledge, there is no evidence of the influence of education on fractional anisotropy.
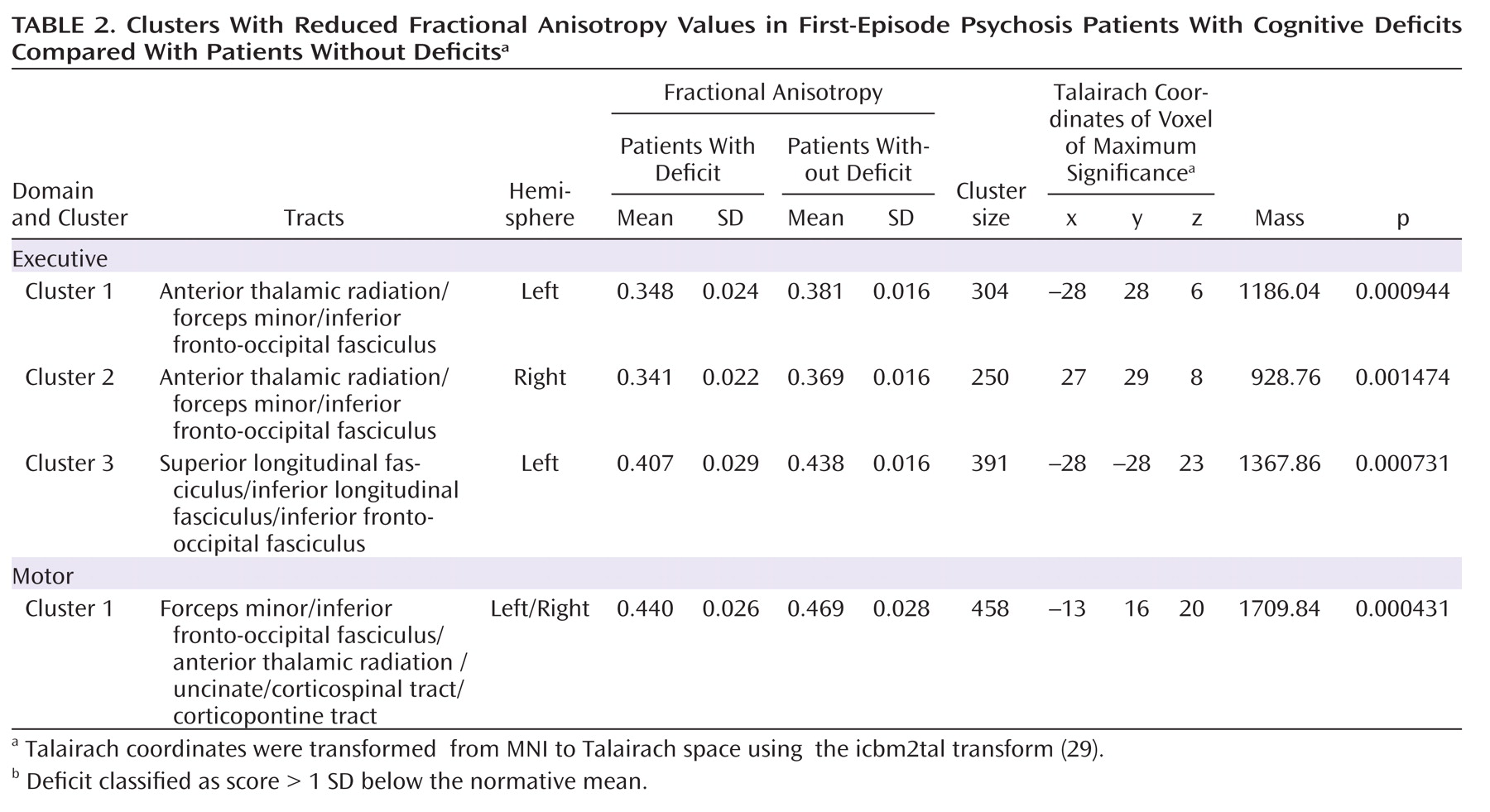
Table 2 shows the four significant clusters identified in the analyses, the size of the clusters, the mean fractional anisotropy, the coordinates of the voxel of maximum significance, and the approximate white matter tracts include in these clusters, identified using the Talairach (
30), Mori et al. (
31), JHU and ICBM_DTI_8 atlases (
32), all integrated into the FSL package http://www.fmrib.ox.ac.uk/fsl/fslview).
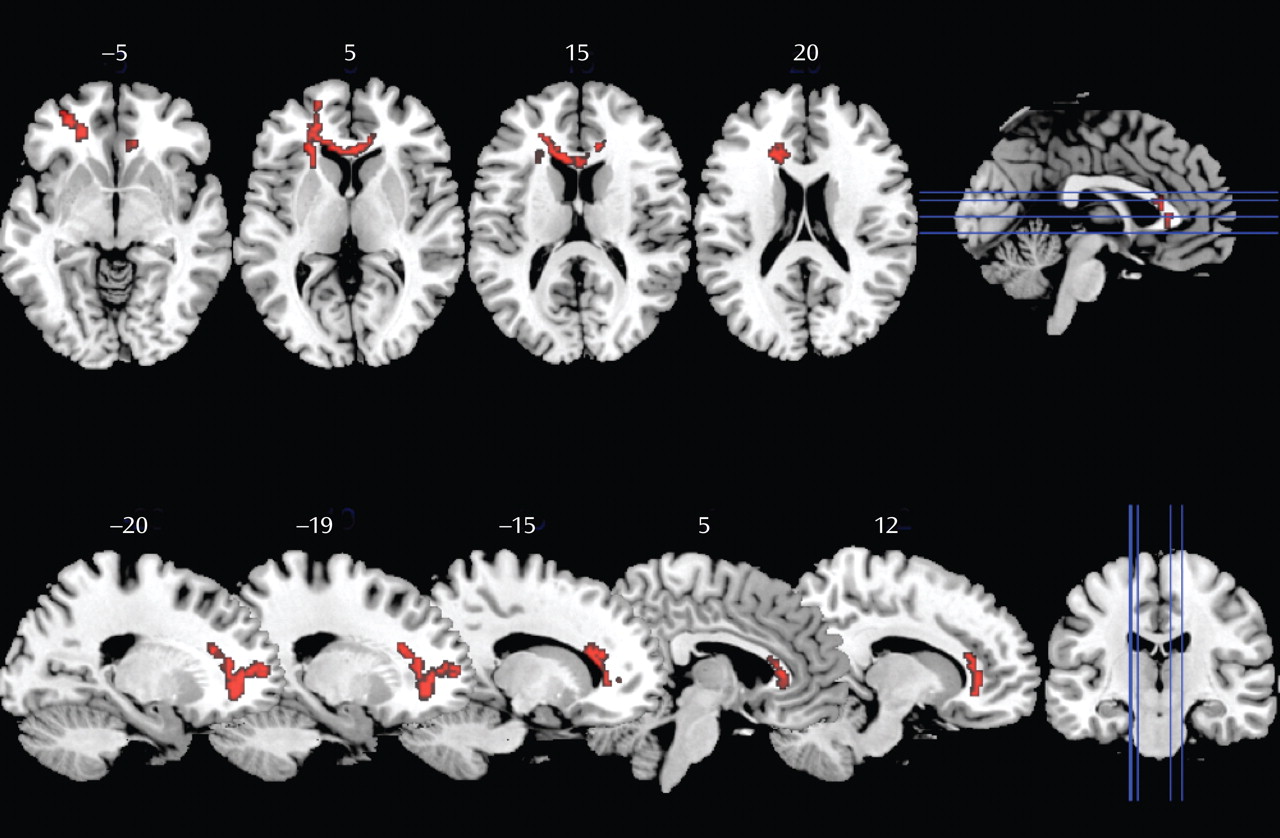
Group comparisons of fractional anisotropy revealed statistically significant lower values in one cluster in the group of patients with impaired motor skills compared with patients without deficit (
Figure 1). This cluster was located in the left frontal white matter. This area mainly corresponds to the forceps minor, the inferior fronto-occipital fasciculus, and the anterior thalamic radiation, although inclusion of fibers of the uncinate, corticopontine, and corticospinal tracts cannot be excluded.
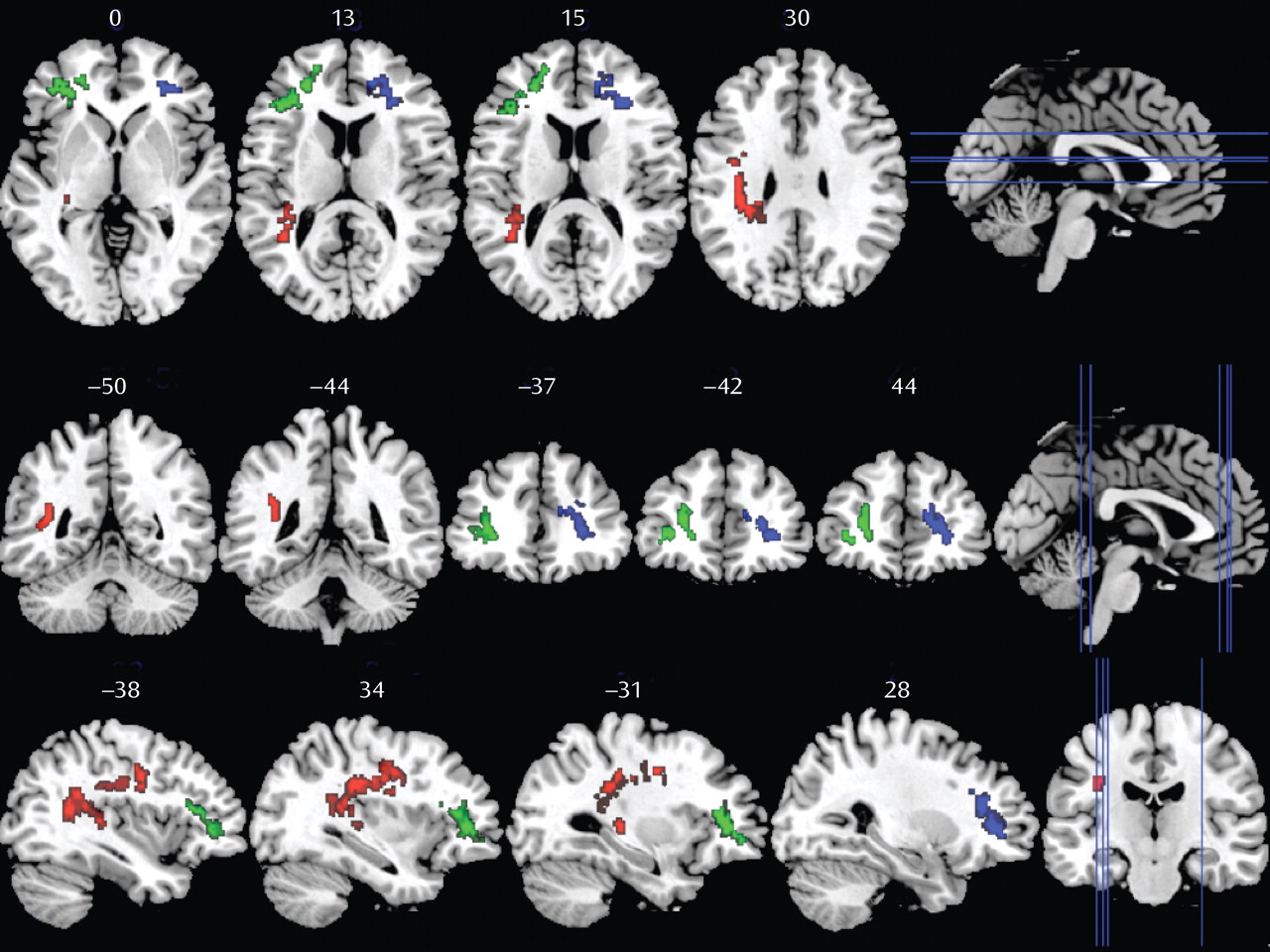
In patients exhibiting executive function impairment, three clusters yielded lower fractional anisotropy values compared with patients without deficit (
Figure 2). Cluster 1 (green) and cluster 2 (blue) include frontal white matter areas corresponding to parts of the left and right anterior thalamic radiation, forceps minor, and inferior fronto-occipital fasciculus. Cluster 3 (red) is located in the left temporal white matter including fibers from the superior longitudinal fasciculus, inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus.
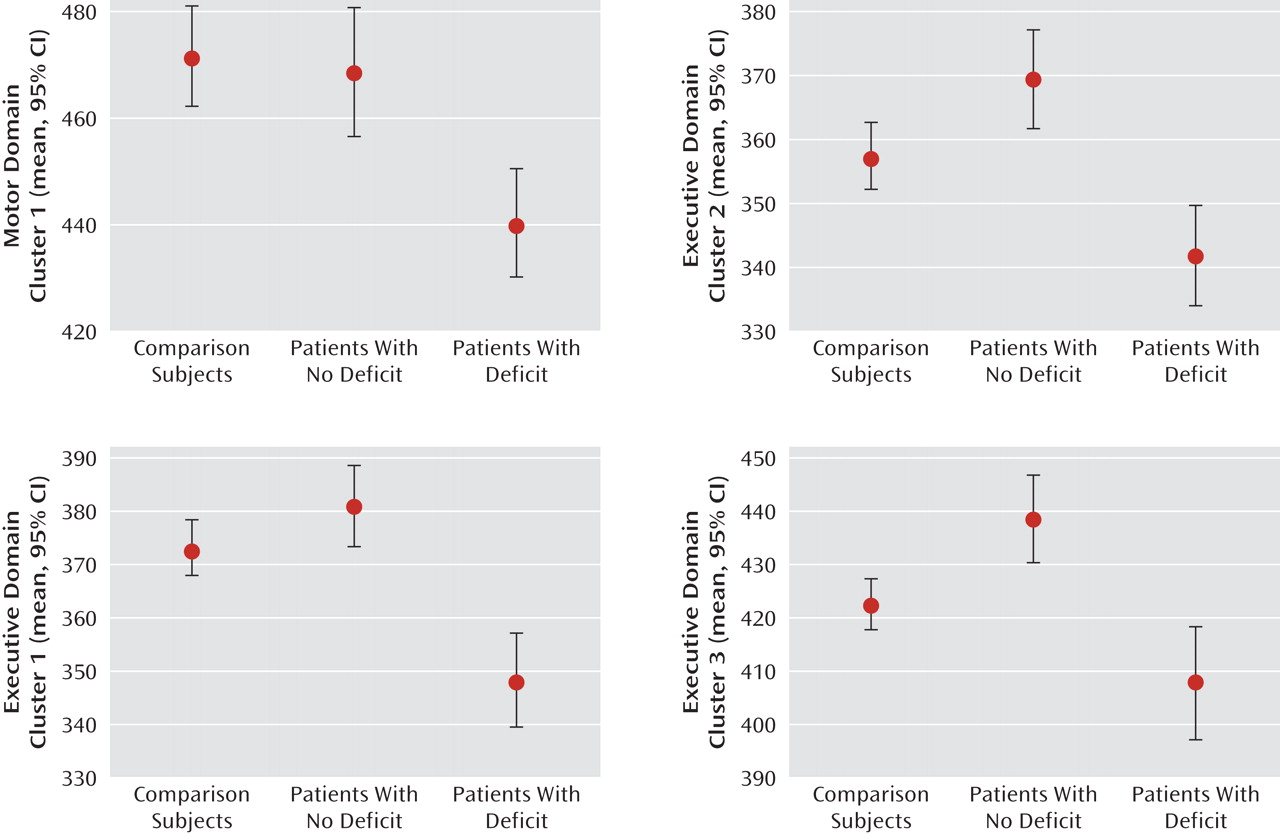
For each individual, patients and comparison subjects, we extracted the mean fractional anisotropy values of these four significant clusters (one of the motor domain comparison and three of the executive domain comparison) identified by the voxel-based morphometry analysis.
Figure 3 demonstrates the overall pattern of fractional anisotropy differences within these regions over all the subject groups.
Voxel-wise diffusion tensor imaging analysis did not show any regions where the values of fractional anisotropy were significantly different when we compared first-episode psychotic patients with poor performance in the attention task or in the verbal memory task with patients who had similar scores to healthy volunteers. There were no clusters in which fractional anisotropy was significantly lower in patients with normal performance compared with patients with poor performance in any of the four cognitive domains.
Discussion
Our data suggest that deficits in executive and motor functioning in first-episode psychotic patients are associated with reductions in white matter integrity in the major fasciculi that connect the frontal and temporal cortices as well as in pathways connecting cortical and subcortical regions. Since the patients had received a mean total of only 35.5 days of antipsychotic treatment, the findings are unlikely to be an effect of medication exposure. No significant correlation was found between years of education and the mean fractional anisotropy values in any of the significant clusters identified for each domain, so it is also unlikely that the results were due to group differences in education.
Fractional anisotropy reductions have been previously associated with cognitive impairments in the context of other disorders. Marenco et al. reported abnormalities in fractional anisotropy in individuals with Williams' syndrome (
9), a genetic disorder with severe visuospatial cognitive deficit. DeLisi et al. (
10) have also reported decreased fractional anisotropy values in Klinefelter's syndrome patients with specific cognitive deficit in executive functioning. Recently, correlations have been reported between fractional anisotropy values and motor skills (pegboard test) in type 1 diabetes mellitus (
11), between fractional anisotropy values in superior longitudinal fasciculus and processing speed in healthy and brain injury groups (
33), and between fractional anisotropy values in the external capsule and inferior-middle superior fasciculus and IQ in children of very low birth weight (
13). Previous studies in schizophrenia have found an association between working memory impairment and reduced fractional anisotropy in the superior longitudinal fasciculus (
14), declarative-episodic verbal memory deficit and lower fractional anisotropy in the uncinate fasciculus (
19), and attentional deficits and lower fractional anisotropy in the cingulum (
34). Collectively, these studies provide evidence that diffusion tensor imaging appears to be a useful tool for assessing the anatomical white matter substrate of neurocognitive dysfunction. However, reduced fractional anisotropy is a nonspecific feature that can result from multiple pathological processes, and the precise relationship between particular cognitive functions and specific white matter tracts is still not well understood.
Our study revealed differences in white matter integrity between patients with and without cognitive deficits. These results are consistent with the findings reported by Skranes et al. (
13), in which adolescents of very low birth weight with low Grooved Pegboard Test performance (one standard deviation below that of healthy subjects) had significantly lower values of fractional anisotropy in several white matter tracts, relative to adolescents of very low birth weight and normal test performance. Our findings are also consistent with those reported by Kraus et al. (
7), in which patients with severe traumatic brain injury showed more regions with significantly lower values of fractional anisotropy than in the group with mild traumatic brain injury. Finally, our results agree with recent data reported by Wexler et al. (
8) in schizophrenia patients, in which the neuropsychologically impaired group (only) had significantly lower white matter volume than healthy subjects. Our findings support the hypothesis proposed by Wexler et al. that these different cognitive phenotypes may correspond with differences in the disease processes.
We failed to find the expected changes in fractional anisotropy in patients with verbal memory and sustained attention deficits. In the case of verbal memory domain, one of the reasons may be the lack of substantial differences in the score between groups. Of the impaired verbal memory group, less than 20% had a z score below two standard deviations. Despite the huge differences in performance between impaired and nonimpaired sustained attention groups, the voxel-wise analysis of this domain also showed no differences in fractional anisotropy. One possible explanation may be that because of the large impairments, the subgroup of patients classified as deficit-free was necessarily small, and thus the analysis may have been underpowered to assess this hypothesis. Another possibility is that attention deficits during the first episode of psychosis might be attributable (at least in part) to transient factors like acute symptoms or medication, and therefore subject to variation over time, and less likely to be related to structural abnormalities.
There are several limitations to our study that should be considered. Voxel-wise analysis involves multiple comparisons, and therefore has the potential risk of both type I and type II error. We sought to reduce these risks by analyzing only the white matter structure (to reduce comparisons at the voxel level, thus improving sensitivity), and by adopting a p value at the cluster level at which we expect less than one false positive over the imaging volume. Another potential limitation may be the error associated with normalization and registration processes. Finally, the voxel-based approach does not allow us to identify precisely which white matter tracts were affected. Future tractography studies should improve the specificity by localizing the fractional anisotropy variations to specific tracts.
The main strength of this study is its relatively large sample size of first-episode patients minimally exposed to antipsychotics. To our knowledge, this is the first study that demonstrates differences in white matter integrity in two different populations of psychotic patients—those with and without impaired cognition.
In conclusion, these findings suggest that moderate to severe deficits in executive and motor functioning are linked to structural deficits in white matter at very early stages of the illness. It should be noted that it was the cognitive impairment itself, and not the illness per se, that was the factor related to abnormalities in white matter structure. Future studies are needed to replicate these findings and to localize the specific tracts affected in different cognitive deficits.