Numerous studies have demonstrated that parental substance use disorders and antisocial behavior are associated with elevated levels of childhood disruptive disorders in offspring. For example, investigations using family, twin, and offspring-of-twin designs have reported that a history of parental alcohol dependence is associated with increased rates of attention deficit hyperactivity disorder (ADHD) (
1), conduct disorder (
2,
3), and oppositional defiant disorder (
3). A number of other studies have also established a link between offspring conduct disorder and parental antisocial behavior and illicit drug abuse (
4–6).
Given the number of parent-child effects, it is reasonable to ask the following question: Are there specific associations between parental substance use disorders and antisocial behavior and childhood disruptive disorders, or can a transmission of some type of general liability account for parent-child resemblance? For example, some well-controlled family and adoption studies have reported specific transmission effects (
2,
4,
7,
8). On the other hand, a growing body of literature supports the notion of a general transmission effect (
4,
9–11).
A conceptual model that can help account for this general transmission effect derives from examining the comorbidity among substance use disorders and antisocial behavior. Specifically, twin studies have demonstrated that the comorbidity among substance use disorders and antisocial behavior can best be accounted for by a highly heritable (approximately 0.80) general liability dimension, typically referred to as the latent externalizing factor
12–18. A similar general factor (albeit somewhat more influenced by shared environmental factors) seems to underlie the comorbidity among childhood disruptive disorders (ADHD, conduct disorder, and oppositional defiant disorder)
19–21. For instance, recent data suggest that symptoms of hyperactivity-impulsivity and oppositionality represent the same underlying liability dimension (
22). It may be that externalizing is a latent, nonspecific liability that accounts for the general transmission effects between parents and children.
One method of disentangling the general and specific effects entails fitting a model that estimates general and specific transmission effects simultaneously. Hicks et al. (
13) tested such a model by utilizing a twin-family design to simultaneously estimate the general and specific transmission effects from parents onto 17-year-old offspring for conduct disorder, adult antisocial behavior (the adult criteria for antisocial personality disorder), alcohol dependence, and drug dependence. Results demonstrated that the transmission of a general externalizing factor liability could account for the parent-child resemblance for the four externalizing disorders and that this general liability was highly heritable. Additionally, Hicks et al. found evidence for specific transmission for conduct disorder, alcohol dependence, and drug dependence, but across siblings rather than from parents to offspring (i.e., horizontal as opposed to vertical transmission). As such, this study provided a model for examining familial transmission in terms of delineating the relative contributions of general and specific effects.
In the present study, we sought to replicate and extend the findings by Hicks et al. by employing a similar family transmission model to clarify the association between substance use disorders and antisocial behavior in parents and childhood disruptive disorders in preadolescent offspring. Specifically, we aimed to determine whether the transmission of the general externalizing factor liability alone could account for parent-child resemblance while also estimating the genetic and environmental contributions to variability in the general and specific transmission effects. We again employed a twin-family design such that each family included both biological parents and a twin pair. However, in contrast to the study conducted by Hicks et al., the twins were from a different cohort and approximately 11 years of age. Moreover, we focused on transmission to childhood disruptive disorders, namely, ADHD, oppositional defiant disorder, and conduct disorder, externalizing disorders that were developmentally appropriate to assess at this age. Additionally, a multi-informant approach (child, mother, and teacher) was used to assess the childhood disruptive disorders. Finally, the sample size for the current investigation included >1,000 families (roughly twice the size employed by Hicks et al.), providing excellent power to detect effects. In line with the findings by Hicks et al., we hypothesized that most of the parent-child resemblance would be mainly accounted for by the transmission of a highly heritable general liability to externalizing psychopathology.
Method
For a full description of the sample recruitment, demographic characteristics, measures, and statistical analysis plan, see the data supplement accompanying the online version of this article. All parents provided written informed consent for themselves and their offspring, and all twins gave written informed assent. The study protocols were reviewed and approved by an internal review board.
Sample
Participants were members of 1,069 families (N=4,276) from the Minnesota Twin Family Study. There were 685 monozygotic twin pairs (male: 52%) and 384 dizygotic twin pairs (male: 49%). The mean age for the twins was 11.8 years (SD=0.4), for the mothers it was 40.2 years (SD=4.9), and for the fathers it was 42.6 years (SD=5.4). Ninety-six percent of the families were Caucasian.
Measures
Parent and child externalizing disorders were assessed via the Substance Abuse Module of the Composite International Diagnostic Interview (
23), a slightly modified version of the Structured Clinical Interview for DSM-III-R-II that includes additional probes to assess the frequency of symptoms for conduct disorder and antisocial personality disorder (
24), and the Diagnostic Interview for Children and Adolescents–Revised (
25), respectively. We assessed parental symptoms of lifetime conduct disorder, adult antisocial behavior, alcohol dependence, and drug dependence. We also assessed symptoms of offspring ADHD, conduct disorder, and oppositional defiant disorder using the Diagnostic Interview for Children and Adolescents–Revised. For twins, a symptom was considered present if it was reported by either the mother or the twin. Finally, teacher ratings of ADHD, conduct disorder, and oppositional defiant disorder were collected for up to three teachers per twin. The mean of the teacher ratings was used for the twin's teacher rating variable, and a composite of the mother, child, and teacher ratings was made for the indices of childhood disruptive disorders by taking a mean of the three reports.
Statistical Analysis
We used structural equation modeling to investigate the link between adult externalizing disorders in parents and childhood disruptive disorders in the preadolescent offspring of these parents as well as the generality versus specificity of transmission effects. The general liability to externalizing disorders was conceptualized as a latent externalizing factor phenotype (
12,
13,
15,
19,
26). For parents, externalizing factor was defined by alcohol dependence, drug dependence, conduct disorder, and adult antisocial behavior and referred to as the externalizing P (parent) factor. For the twins, externalizing factor was defined by ADHD, conduct disorder, and oppositional defiant disorder and referred to as the externalizing O (offspring) factor. Symptom counts were used for all disorders. General transmission effects were operationalized as the latent correlations between the externalizing P and O phenotypes. We estimated the specific transmission effects of a given disorder by allowing the residual variance (i.e., variance unaccounted for by externalizing factors) of the parental symptom count variables to covary with the residuals of the twins' symptom count.
We also used standard biometric models to examine the influence of additive genetic, shared environmental, and nonshared environmental influences (the ACE model) on the externalizing O factor as well as on each childhood disruptive disorder. The additive genetic component (a2) refers to the effect of individual genes summed over loci on trait variance. Genetic influences are inferred if the monozygotic correlation is greater than the dizygotic correlation for a given trait. Shared environmental (c
2) effects refer to environmental influences that increase similarity between members of a twin pair. Shared environmental effects are inferred if the dizygotic correlation is more than one-half the monozygotic correlation. Nonshared environmental (e
2) effects refer to environmental factors that contribute to differences between members of a twin pair. Measurement error is also included in the estimate of e
2. Additionally, if the monozygotic correlation is more than one-half the dizygotic correlation, it is common practice to investigate nonadditive genetic (dominance [d
2]) models as an alternative to ACE models. Because c
2 and d
2 are estimated using the same information, the two parameters cannot be estimated simultaneously. Biometric analyses were conducted using the Mx computer program (
27).
Model fitting for family transmission effects was conducted in Mplus 5.0 (
28) using a robust maximum-likelihood estimator, which is appropriate when analyzing non-normal variables. The non-normality was the result of positive skew in the symptom count variables, which is typical for population-based samples. The fit of models was evaluated using the mean-adjusted (Satorra-Bentler) chi-square fit statistic (
29), the Bayesian information criterion (χ
2–df [ln N]) (
30), and the root mean square error of approximation (
31). The mean adjusted chi-square provides an overall estimate of model fit for non-normal data. For nested models, the difference in chi-square can be used to determine whether additional parameters significantly improve the model fit. Because we conducted 27 tests of specific transmission, we used a Bonferroni-corrected alpha of 0.0019 (0.05/27) when comparing Δχ
2 for nested models. The Bayesian information criterion is a function of a model's chi-square value and degrees of freedom and penalizes the model fit for the retention of unnecessary parameters. This fit index is not interpreted in isolation; rather, it is used to compare alternative models such that lower Bayesian information criterion scores are indicative of a better fit. When comparing models, a difference in Bayesian information criterion of 0–2 is considered weak evidence in support of the model with the lower criterion value, a difference of 2–6 is considered positive evidence, a difference of 6–10 is considered strong evidence, and a difference >10 is considered very strong evidence (
32). The root mean square error of approximation provides an estimate of discrepancy in the model fit per degree of freedom, with values of 0.08 indicating a good fit and ≤0.05 indicating a very good fit to the data (
31).
Results
General Versus Specific Transmission of Childhood Disruptive Disorders
Results of the model fitting are provided in
Table 1. An initial model that allowed for only general transmission of externalizing disorders from parents to offspring provided an adequate fit to the data (χ
2=699.96, df=189, Bayesian information criterion=−618.22, root mean square error of approximation=0.071). This model served as the comparison model for all subsequent analyses.
To test for disorder-specific effects, we allowed the residual variances of parental disorders to covary with the residual variances of offspring disorders (e.g., mother conduct disorder and twin conduct disorder, father alcohol dependence and twin ADHD). If the model fit improved, this indicated that parents and offspring were more similar than would be expected given only general transmission. To maximize power to detect specific effects, we conducted one degree of freedom test separately for each paternal and maternal disorder (
Table 1), which is differentiated from the general transmission only model by a reduction of one degree of freedom and subsequent changes in model fit indexes. We conducted 24 tests to determine whether a specific effect model supporting parent-offspring transmission fit better than the general transmission model. None of the specific transmission effects resulted in a significant reduction in chi-square (Bonferroni corrected-alpha <0.0019), a drop in Bayesian information criterion of at least two (rather, almost all specific effects resulted in higher Bayesian information criterion values), or a lower root mean square error of approximation value. Therefore, the results show that a general transmission only model can account for familial resemblance in adult externalizing disorders in parents and childhood disruptive disorders in their offspring.
Next, we examined specific cross-twin (sibling) effects for ADHD, conduct disorder, and oppositional defiant disorder by allowing the residual variance of each disorder to correlate across members of the twin pair. We allowed the paths to vary by zygosity, and therefore there were two degree of freedom tests for each disorder. Cross-twin specific effects were detected for each childhood disorder (
Table 1), indicating familial liability factors to these disorders that are independent of the general liability but that do not stem from parental externalizing disorders.
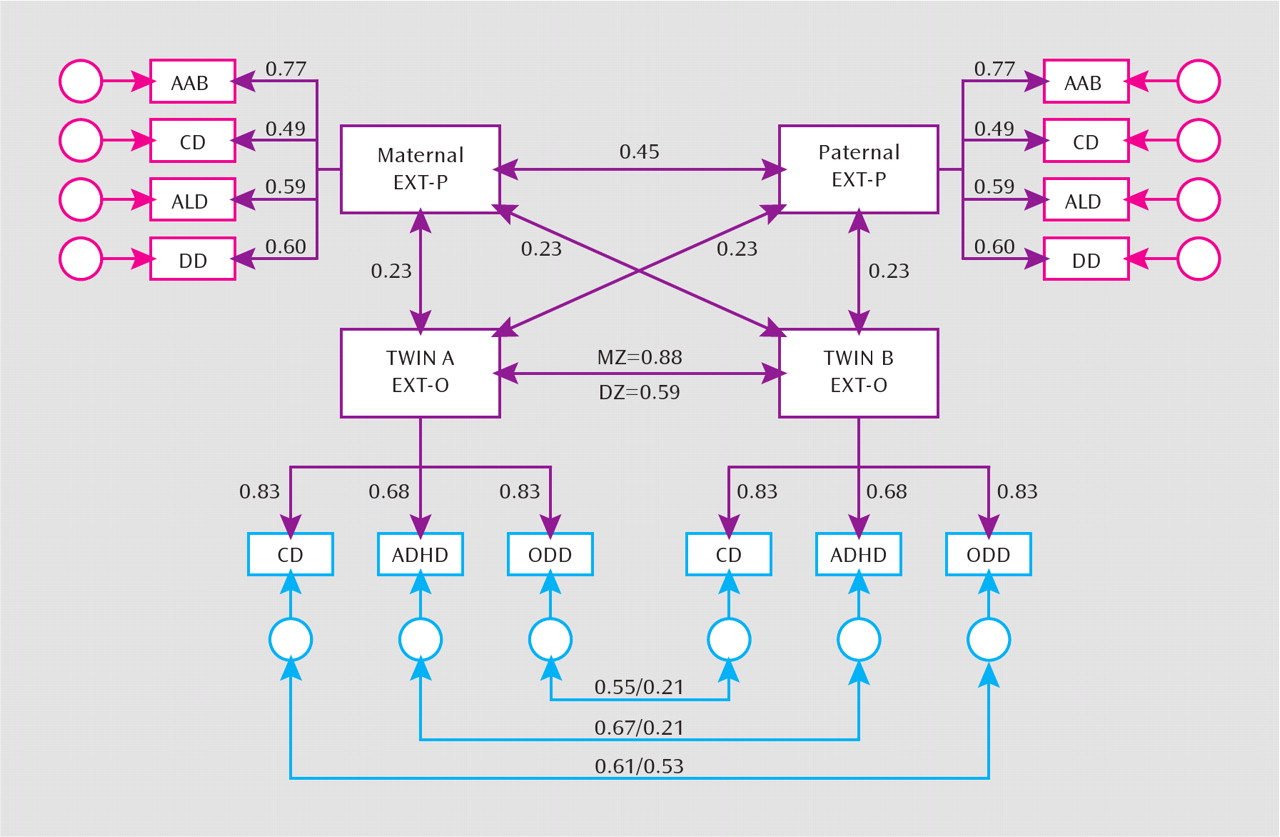
Maternal and paternal general transmission effects were estimated as the latent correlation between externalizing P and O factors. The latent correlations between the mother and father P factors and the O factor were r=0.26 (95% confidence interval [CI]=0.13−0.38) and r=0.20 (95% CI=0.12–0.29), respectively. As such, in the next model, we equated the effects of the externalizing P factor on the externalizing O factor. Equating the parental effects yielded a lower Bayesian information criterion value, indicating that the strength of maternal and paternal transmission was not significantly different. The resulting latent correlation between the externalizing P and O factors (r=0.23; 95% CI=0.14–0.31) was moderate and represents the effect of the general transmission of parental externalizing disorders on offspring childhood disruptive disorders (
Figure 1).
To ensure that the parental transmission effects did not differ by the gender of the offspring, we conducted the same tests of specific and general transmission effects (i.e., following the aforementioned procedure and models) separately for male and female twins. The general transmission effects could be equated for male and female twins without a loss in model fit. Moreover, we did not detect any specific transmission from parents to offspring for either male or female twins. To ascertain that these results did not differ by informant, we fit separate models using parent, child, and teacher reports for offspring disorders. The results were consistent with those shown in
Figure 1.
Genetic and Environmental Contributions to Variation in Externalizing Factors and Disorder-Specific Liabilities
In testing the magnitude of genetic influences on cross-twin effects, we first examined the latent correlation between the mother and father externalizing P factors. The correlation between parents sometimes increases the similarity of dizygotic twins in twin studies, thereby decreasing the study heritability (
33). There was a significant correlation between the maternal and paternal externalizing factors (r=0.45; 95% CI=0.31–0.60). Despite this finding, the cross-twin correlations for the externalizing O factor differed strongly across zygosity, indicating a strong genetic influence. However, the dizygotic correlation was greater than one-half the monozygotic correlation, suggesting shared environmental effects on the general externalizing O factor. For the disorder-specific effects (
Figure 1), the monozygotic correlations for ADHD and oppositional defiant disorder were larger than the dizygotic twin correlations, indicting specific genetic effects. In contrast, the monozygotic and dizygotic correlations were quite similar for conduct disorder, indicating a shared environmental effect.
Next, we fit biometric models to decompose the variance of each disorder into its additive genetic (a
2), shared environmental (c
2), and nonshared environmental (e
2) factors (
Table 2). Genetic effects were large for ADHD and oppositional defiant disorder and moderate for conduct disorder. Notably, because the monozygotic correlation for ADHD was more than one-half the dizygotic correlation, we also investigated a nonadditive genetic model, with dominance (d
2) replacing the c
2 effect. This model did not fit significantly better than the ACE model (change in Bayesian information criterion was only –1.62), and dominance was not significant (d
2=0.27; 95% CI=0.00–0.60). Thus, an ACE model was fit for each disorder. Only conduct disorder evidenced a notable shared environmental component. Small nonshared environmental effects were detected for each disorder. Finally, we examined the generality and specificity of the genetic and environmental effects on the three childhood disruptive disorders by fitting a biometric factor model (
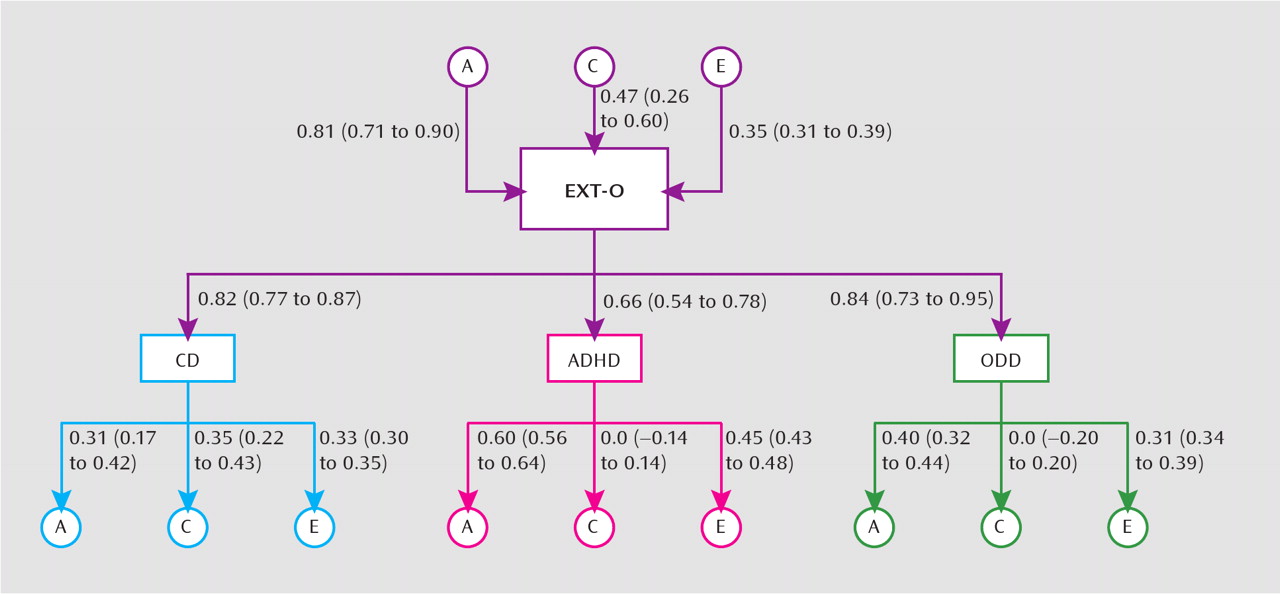
Figure 2). The general externalizing O factor was primarily influenced by genetic factors (a
2=0.65; 95% CI=0.51–0.81) but also exhibited a notable shared environmental component (c
2=0.23; 95% CI=0.07–0.36), with the remaining variance as a result of nonshared environmental effects (e
2=0.12; 95% CI=0.10–0.15). This indicates largely common genetic factors underlying the comorbidity among the childhood disruptive disorders. We also detected several specific ACE effects on the individual disorders. For each disorder, we detected specific genetic effects. These effects were large for ADHD (a
2=0.36; 95% CI=0.31–0.41), moderate for oppositional defiant disorder (a
2=0.16; 95% CI=0.10–0.19), and modest for conduct disorder (a
2=0.10; 95% CI=0.03–0.18). Only conduct disorder exhibited a strong shared environmental effect (c
2=0.12; 95% CI=0.05–0.18). Last, all three disorders exhibited moderate specific nonshared environmental effects (ADHD: e
2=0.20; 95% CI=0.18–0.23; oppositional defiant disorder: e
2=0.14; 95% CI=0.12–0.15; conduct disorder: e
2=0.11; 95% CI=0.09–0.12).
Discussion
We examined whether the link between parental substance use disorders and antisocial behavior and childhood disruptive disorders in the offspring is a result of the transmission of a general liability to multiple disorders or disorder-specific liabilities. Consistent with previous findings for the transmission of adult externalizing disorders to offspring in late adolescence (
13), the present results indicated that a general transmission factor, externalizing, accounted for the co-occurrence of adult externalizing disorders in parents and childhood disruptive disorders in their offspring. In other words, parents pass on a general liability to externalizing psychopathology that is then manifested in their offspring in the form of attention problems, hyperactivity, oppositionality, and conduct problems. We also detected disorder-specific effects that were present across siblings rather than transmitted from parents to their children. Together, these results provide a conceptual model for the transmission of externalizing from parents to their offspring that accounts for the link between adult and child externalizing psychopathology.
We also estimated the genetic and environmental contribution to variations in both the general and specific liabilities to childhood disruptive disorders. The general externalizing O liability was highly heritable, indicating that common genetic risk factors predominately account for the comorbidity among childhood disruptive disorders. However, the externalizing O factor also exhibited a moderate shared environmental effect, indicating that certain environmental influences that increase twin similarity also account for the co-occurrence among childhood disruptive disorders. Thus, our results are consistent with previous work with adolescent and adult samples that have implicated a highly heritable externalizing factor (
15,
34,
35) as well as with studies (and a recent meta-analysis) utilizing samples of children and reporting a strong influence of shared environmental effects (
26,
36).
One notable finding concerns the large specific genetic effect on ADHD, suggesting that there are substantial genetic factors contributing to the variation in this disorder that are independent of the general liability to the externalizing O factor. This effect is not unique, since similar findings have been reported previously (
37). This effect likely stems from the content differences between ADHD versus conduct disorder and oppositional defiant disorder. In particular, the latter disorders involve explicit violation of norms or rules, the rights others, and disobeying authority. ADHD, on the other hand, does not involve the violation of others' rights; rather, much of the content focuses on the child being difficult to manage and instruct. In this way, the content differences between the disorders might account for the specific genetic effect in ADHD.
Another notable finding was that shared environment influenced conduct disorder alone. The robust shared environmental influence on conduct disorder has been reported previously (
21,
38). However, shared environmental influences on antisocial behavior seem to decline with age such that antisocial behavior in late adolescence and adulthood is influenced mainly by genetic factors (
39,
40). Thus, our results revive the importance of examining shared environmental variables as processes underlying the comorbidity among childhood disruptive disorders.
Some limitations of the present study should be noted. First, the cross-sectional nature of the study precludes any inferences regarding generalizability of these findings at different ages. Second, the study utilized a homogenous sample of mostly Caucasian families. Third, although there was evidence for a correlation between paternal and maternal externalizing, there was no way of determining whether this correlation reflected a tendency of like individuals to mate with each other (assortative mating) or the tendency for spouses to become more similar with time. Fourth, although less of a limitation than a future direction, the present study did not examine the exact nature of the environmental influences on externalizing, such as socioeconomic status or neighborhood disruption, which could also have moderated the genetic effects on externalizing. The identification of the role of environmental factors may add precision to future work. Finally, we utilized a standard additive ACE model to estimate the effects of genes and environment. However, recent investigation focused on the genetics of externalizing behavior suggests the presence of gene-by-environment interactions as well. Future models following the present research will benefit from testing this model with and without the presence of gene-by-environment interaction effects.
To conclude, our results provide important insight into the familial aggregation of externalizing disorders. Specifically, they indicate that a focus of research should be to identify the common risk factors across disorders because this general liability accounts for the link between substance use disorders and antisocial behavior in parents and childhood disruptive disorders in their offspring. The knowledge of the general transmission effect and support for a common liability for childhood disruptive disorders might be of interest to clinicians as well. In particular, these results suggest that these common—more so than specific effects—should be the targets of prevention and intervention efforts.
These results also provide insight regarding how genetic and environmental factors can change as a function of developmental context. Although genetic factors are large and present throughout development, shared environmental factors are major contributors to the variation in the co-occurrence of externalizing behaviors in childhood (
36), whereas in late adolescence and adulthood this is not necessarily true. A potential mechanism for this shift may be a transition from passive gene-environment correlation processes in childhood to evocative and active gene-environment correlation processes in adolescence and adulthood. Further research that integrates the current findings within a developmental context with a focus on delineating these mechanisms of gene-environment interplay will yield insights into the etiology of disruptive disorders. Such knowledge can inform intervention strategies to help alleviate the stress experienced by individuals and families.