Epidemiological data suggest that borderline personality disorder has a lifetime prevalence of 1% –5% (
1), with about 75% of clinical subjects being female (
2). Patients with borderline personality disorder often have axis I and axis II comorbidity and high disability levels, and their utilization of medical resources is disproportionate to their numbers (
3).
Borderline personality disorder is characterized by dysregulation of emotion processing, manifested as affective lability and impulsive behaviors, including aggression and self-harm (
4). This dysregulation is exemplified by short-duration, often severe, rapidly changing mood states that are highly reactive to environmental stimuli (
5–
7). Patients appear to react more quickly, with greater intensity and a slower return to baseline state (
7).
Borderline personality disorder exhibits some clinical and biological similarities to major depressive disorder, but available evidence suggests that the two have distinct pathophysiological profiles (
8). While patients with major depression display a negative emotional bias and difficulties in shifting emotional states, those with borderline personality disorder lack effective regulation of responses to emotionally salient stimuli (
5,
6). Whether development of borderline personality disorder depends on genetic factors alone or is a variable combination of genetic vulnerability and environmental factors (
4), exploration of the underlying neurobiology of the disorder would provide significant insight into its pathophysiology.
Resting metabolic rate reductions in the prefrontal cortical regions of patients with borderline personality disorder have been observed in some but not all studies (
9,
10), as well as reductions in the cuneus and hippocampus and increases in the cingulate and frontal gyri relative to matched comparison subjects (
11). Reductions in baseline metabolic function in the posterior cingulate cortex, temporal lobe, and precuneus have been noted in patients with both borderline personality disorder and posttraumatic stress disorder (
12). Patients with borderline personality disorder and a history of childhood abuse have been found to display reductions in amygdala and hippocampal volumes (
13). The relationship between metabolic activity of the prefrontal cortex and the amygdala was diminished in a sample of impulsive-aggressive borderline personality disorder patients relative to comparison subjects, supporting a hypothesis of poor prefrontal control of emotion-regulatory limbic structures (e.g., the amygdala) in these patients (
14,
15). Challenge studies employing fMRI and emotional stimuli support this hypothesis. Reductions in cingulate and prefrontal cortical function and increases in amygdala activity were found in the presentation of fear faces and in attempted inhibition of negative emotional stimuli (
16,
17).
In this study, we explored hypothesized neurochemical mechanisms in borderline personality disorder pathophysiology. The endogenous opioid system and μ-opioid receptors have long been implicated in emotional and stress response regulation. In animal models, reductions in endogenous opioid system function are associated with attachment behavior deficits and anxiety-like responses (
18), and in humans, with normal and pathological (e.g., major depressive disorder) emotion regulation (
19, 20), in addition to the system's traditional role in modulating responses to physical and emotional stressors (21, 22). Pain thresholds are found to be increased in borderline personality disorder and associated with dissociative symptoms and negative affect (23). In an fMRI study, intensity-matched pain stimuli induced greater activity in the prefrontal cortex but lower activity in the amygdala and anterior cingulate of patients with borderline personality disorder relative to comparison subjects. These are regions interfacing emotion, pain, and stress regulation and where μ-opioid receptors have important modulatory roles (22, 23). These regions and systems also have been found to be related to trait impulsivity in healthy volunteers in response to a pain stressor (24).
We used positron emission tomography (PET) and a selective μ-opioid receptor radiotracer to examine availability (nondisplaceable binding potential, or BPND) of these receptors under neutral (baseline) conditions and during induction of a sustained sadness state in female borderline personality disorder patients and matched comparison subjects (19, 20). Under challenge conditions, acute reductions in BPND are thought to reflect activation of μ-opioid-receptor-mediated neurotransmission (e.g., competition of the endogenous ligand with the radiotracer, receptor activation and recycling). Increases in BPND would then reflect deactivation of neurotransmission (19, 20). We hypothesized that differences between the borderline personality disorder and comparison groups would include greater baseline μ-opioid BPND in emotion processing regions in the borderline personality disorder group, reflecting chronic lower levels of opioid regulatory control with compensatory receptor up-regulation. During sustained sadness we expected an exaggerated response in these same areas, reflecting high sensitivity of borderline personality disorder patients to emotional stimuli, with greater activation of the stress regulatory endogenous opioid system and μ-opioid receptors.
Method
Participants
Participants were 18 right-handed female patients with borderline personality disorder (mean age=28 years [SD=9], mean educational level=14 years [SD=2]) and 14 age-matched healthy female comparison subjects (mean age=35 years [SD=10], mean educational level=17 years [SD=2]). Nine comparison subjects were in a previous study (19), and nine in a second study (20), with an overlap of 10 comparison subjects between the present study and previously published work; four comparison subjects were unique to this study. Axis I and axis II diagnoses were made via the Structured Clinical Interview for DSM-IV Axis I Disorders (25) and the Structured Clinical Interview for DSM-IV Axis II Disorders (26) and confirmed by an experienced clinician (K.R.S.). Patients with borderline personality disorder also met the DSM-IV borderline personality disorder criterion of affective instability. Exclusion criteria were concurrent axis I and III diagnoses (except for mood disorder); history of psychosis or head trauma; and current or recent (within 3 months) illicit substance use, abuse, or dependence. All participants were medication free for at least 3 months. Comprehensive urine drug screens were negative prior to the PET scan.
Participants completed the NEO Personality Inventory–Revised (NEO-PI-R; 27), the Barratt Impulsiveness Scale (28), and the Dissociative Experiences Scale (29) prior to scanning. To reduce phenotypic and neurobiological variability, only women were studied (30).
All participants provided written informed consent. Protocols were approved by the Investigational Review Board and the Radioactive Drug Research Committee at the University of Michigan.
Induction of Sustained Affective States
Neutral and sadness states were randomized, counterbalanced, and initiated either 5 minutes or 45 minutes after administration of the radiotracer. During the neutral condition, participants were instructed to try to create passive relaxation while avoiding any active cognitive process. During sadness induction, participants recalled a previously rehearsed past autobiographical vignette associated with sadness. Both states were practiced prior to the actual scan and the specific events recorded. Participants rated their experience every 10 minutes on the sadness subscale of the Positive and Negative Affect Schedule (PANAS; 31) to ascertain maintenance of their emotional state; the subscale includes the adjectives "sad," "blue," "downhearted," "alone," and "lonely." The full PANAS (60 adjectives, 20 of which are grouped into two main affective subscales, positive and negative) was completed by participants at baseline and after completion of neutral and sadness states (45 minutes and 90 minutes after administration of the radiotracer).
Neuroimaging Methods
One PET scan was acquired over 90 minutes with a Siemens (Knoxville, TN) HR+ scanner in three-dimensional mode (reconstructed full width at half maximum resolution, ∼5.5 mm). [
11C]carfentanil, a selective μ-opioid receptor radiotracer, was administered at a dose of 10–15 mCi and mass <0.03 μg/kg (tracer quantities). Acquisition, reconstruction, and emission image coregistration protocols were identical to those used in previous studies (
19,
20).
Image data were transformed on a voxel-by-voxel basis into two sets of parametric maps: a tracer transport measure (K
1 ratio) and receptor-related measures during neutral and sadness states. To avoid arterial blood sampling, these measures were calculated employing a modified Logan graphical analysis (
32) using the occipital cortex (an area devoid of μ-opioid receptors) as the reference region. The slope of the Logan plot is proportional to [(B
max/K
d) +1] for this receptor site (B
max= receptor concentration; K
d= receptor affinity for the radiotracer); B
max/K
d is the "in vivo receptor availability" measure, or nondisplaceable binding potential, BP
ND. With the bolus-continuous infusion protocol, the Logan plot becomes linear approximately 4–6 minutes after administration of the radiotracer, allowing for quantification of receptor sites. BP
ND values for neutral and sadness states were calculated from data from 10–45 minutes and 50–90 minutes after radiotracer administration. Anatomical MRI scans were acquired on a GE 3-T scanner (General Electric, Milwaukee). Acquisition sequences were axial spoiled gradient-recall acquisition (echo time=3.4 msec, repetition time=10.5 msec, inversion time=200 msec, flip angle =25°, number of excitations=1, using 124 contiguous images 1.5 mm thick). K
1 and BP
ND images for each experimental period and MR images were coregistered to each other and to the Montreal Neurological Institute (MNI) stereotactic atlas orientation (
33). Statistical parametric maps of differences between conditions were generated by anatomically standardizing the T
1 spoiled gradient-recall acquisition MRI of each participant to the MNI stereotactic atlas coordinates, with subsequent application of this transformation to the receptor binding maps. The accuracy of coregistration and nonlinear warping algorithms was confirmed for each participant by comparing the transformed MRI and PET images to each other and to the atlas template.
Data Analysis
Differences between groups and conditions were calculated with subtraction analyses and mapped into stereotactic space with t maps of statistical significance using the SPM2 (Wellcome Department of Cognitive Neurology, University College, London) and MATLAB (MathWorks, Natick, Mass.) software packages with a general linear model and correction for multiple comparisons. No global normalization was applied to the data; calculations presented are based on absolute BP
ND estimates. Only regions with specific μ-opioid receptor binding were included in the analyses (voxels with BP
ND values >0.1). Subtraction analyses were performed on μ-opioid BP
ND images separately to assess main effects. For each subtraction analysis, one-sample paired t values were calculated for each voxel using pooled variance across voxels. Significant differences were detected using a statistical threshold of p<0.0001 for regions known to be involved in opioid modulation of affective and stress responses (the rostral anterior cingulate, medial prefrontal and orbitofrontal cortex, inferior temporal cortex, insula, posterior thalamus, nucleus accumbens/ventral pallidum, and amygdala [19, 20]). Statistical thresholds for other regions were corrected for type I error rate at p=0.05 for multiple comparisons (
34). The BP
ND values were extracted from image data by averaging the voxel values contained in an area where significant differences were obtained down to a threshold of p<0.01. These values were then used to plot the data and perform correlation analyses, ruling out the presence of outliers. Planned analyses included Spearman rank correlations between regional BP
ND values or sadness-induced changes in BP
ND and the NEO-PI-R neuroticism subscore, the Barratt Impulsiveness Scale score, and the Dissociative Experiences Scale score and change in PANAS scores at p<0.05 with subsequent Bonferroni corrections for multiple comparisons.
Results
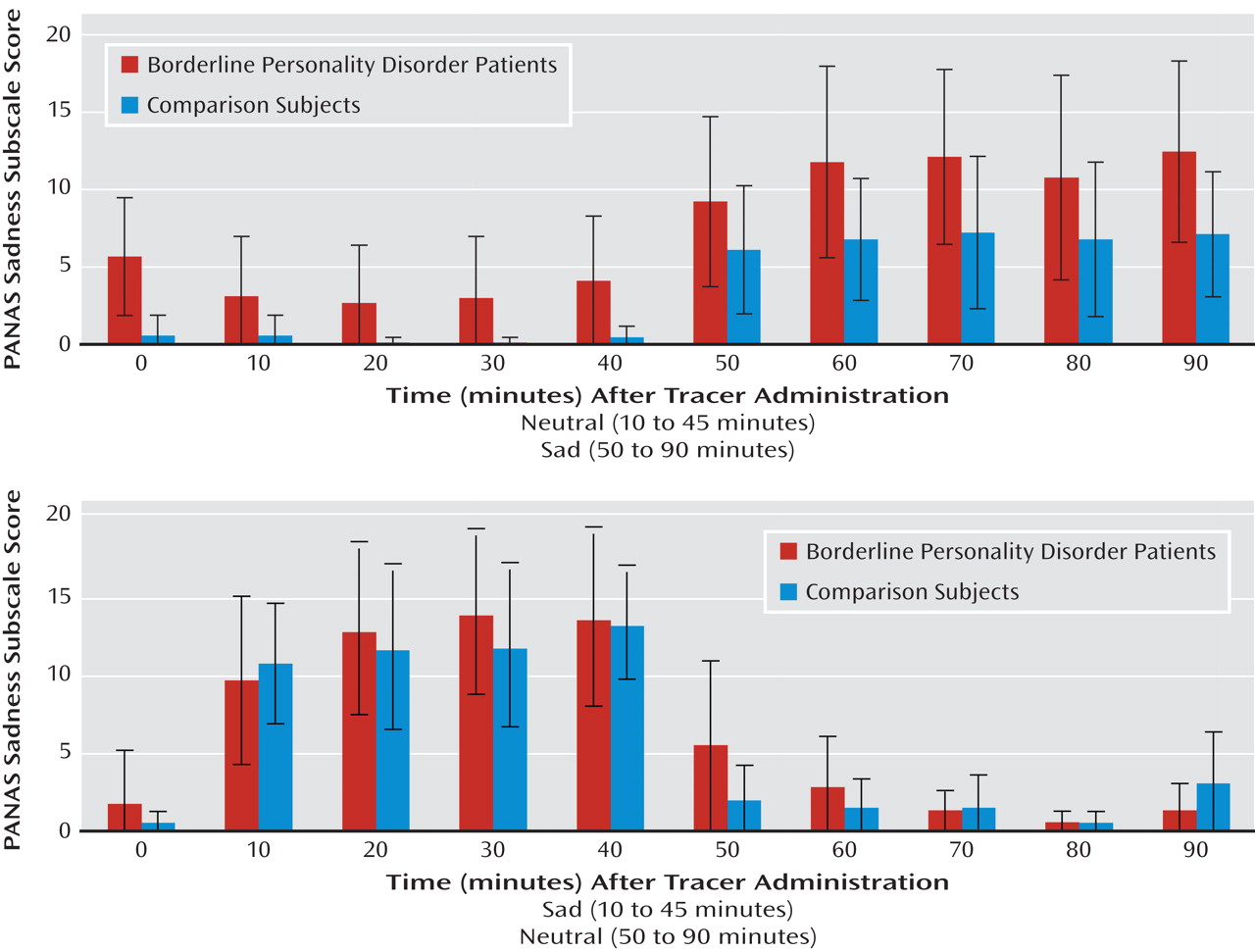
Sadness was maintained through the induced sadness experimental period in both borderline personality disorder patients and comparison subjects (
Figure 1). Repeated-measures analysis of variance (ANOVA) showed significant effects of experimental condition (sadness > neutral [df=7, 1, 63; F=112.2, p<0.0001]) and an interaction between experimental condition and order (F=9.0, p=0.006), with greater negative affect scores when sadness induction was performed first. An effect of diagnosis was observed, with the borderline personality disorder group showing greater PANAS sadness subscale scores (F=4.2, p=0.05).
PANAS negative affect ratings acquired after scan completion showed a mean score of 13 [SD=4] in the neutral state and 19 [SD=7] in the sadness state in the comparison group and 14 [SD=5] in the neutral state and 25 [SD=7] in the sadness state in the borderline personality disorder group. Repeated-measures ANOVA showed significant effects of condition (sadness > neutral [df=7, 1, 63; F=40.8, p<0.000]) and diagnosis (borderline personality disorder group > comparison group [F=5.5, p=0.03]). Order effects did not achieve statistical significance (F=3.8, p=0.06).
Baseline Measures
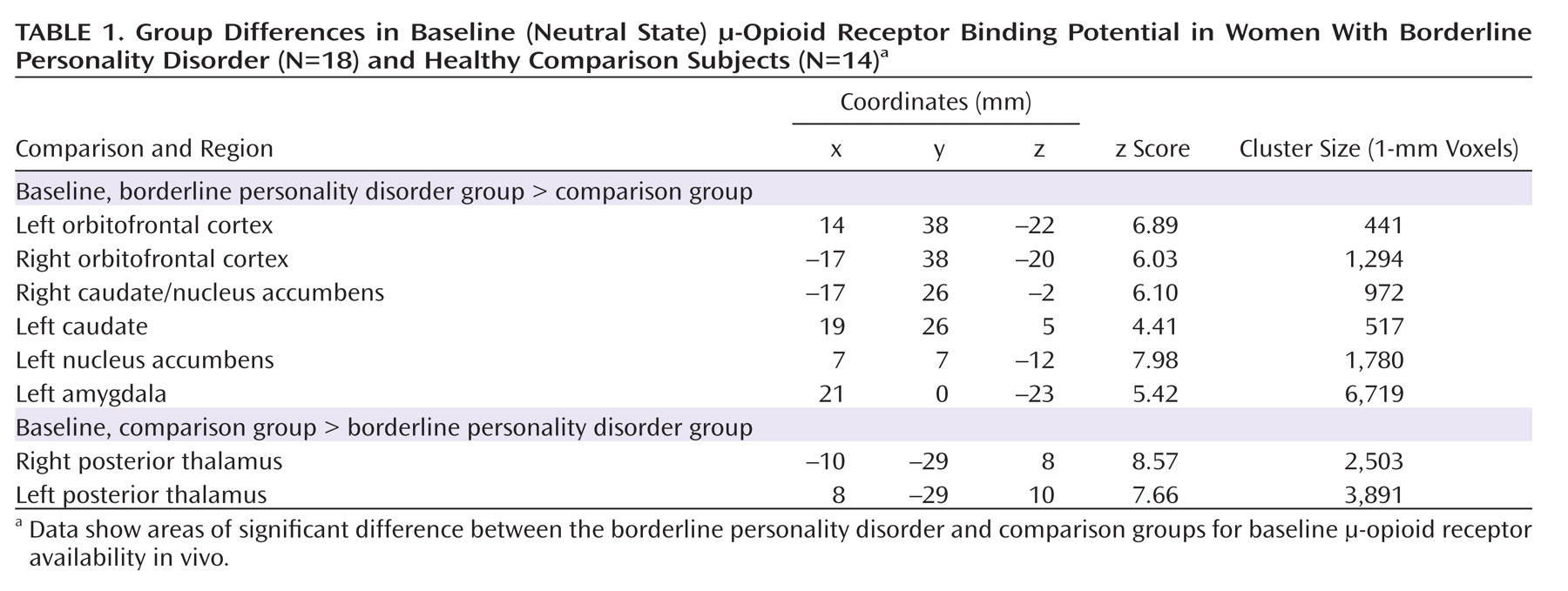
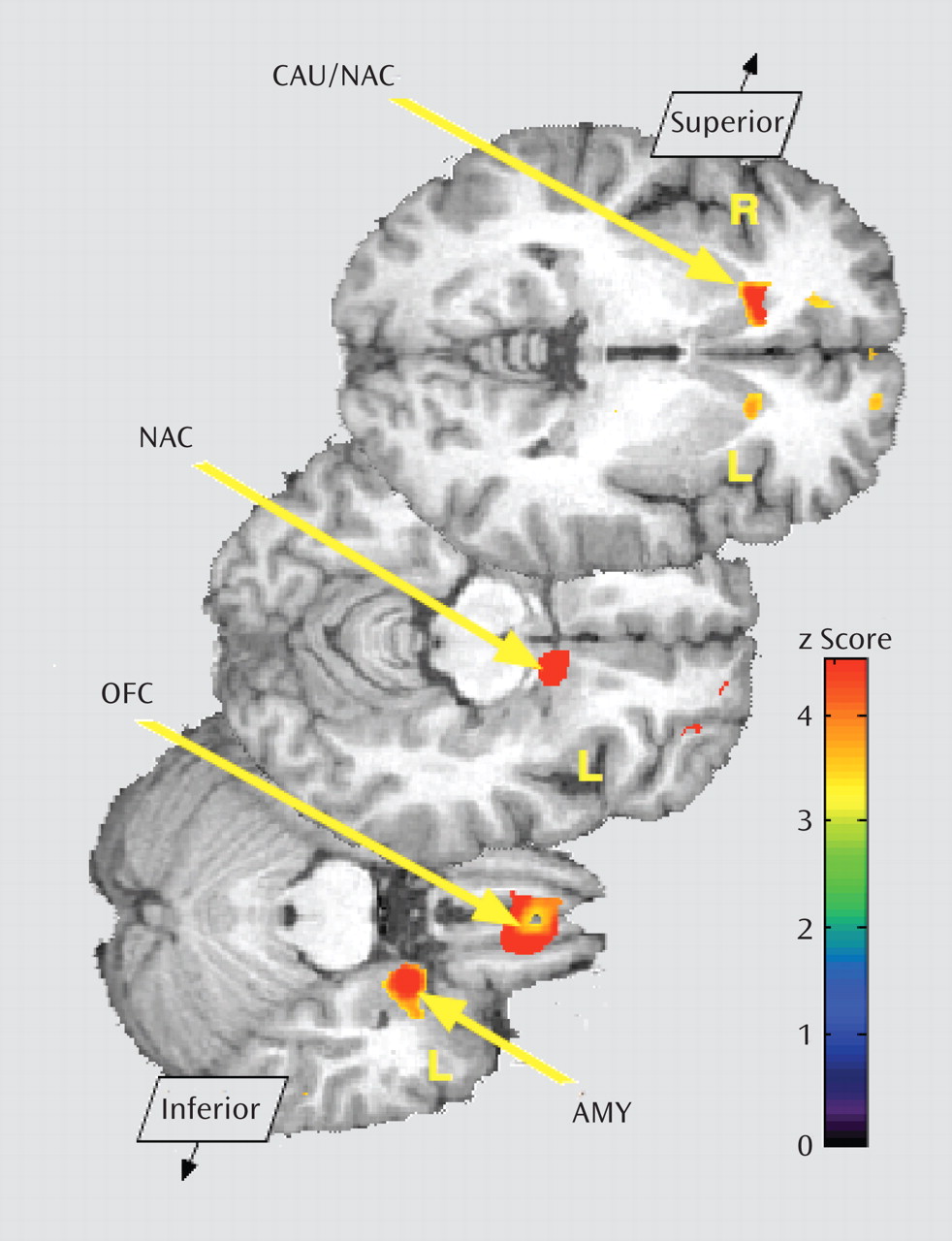
Baseline data (neutral state) comparisons between groups showed significantly greater μ-opioid BP
ND in borderline personality disorder patients in the orbitofrontal cortex bilaterally; the caudate nucleus bilaterally, extending into the nucleus accumbens on the right; the left nucleus accumbens; and the left amygdala (
Table 1,
Figure 2). These corresponded to average between-group differences of 44% and 47% for right and left orbitofrontal cortex, respectively; 44% and 32% for right and left caudate, respectively; 25% for left nucleus accumbens; and 35% for left amygdala. The comparison group demonstrated greater BP
ND in the posterior thalamus bilaterally (Table 1), corresponding to group differences of 39% on the right and 37% on the left.
No significant correlations were obtained between Barratt Impulsiveness Scale scores or NEO-PI-R neuroticism subscale scores and baseline regional μ-opioid BPND. Dissociative Experiences Scale scores were negatively correlated with BPND in the right caudate region (r=–0.57, p=0.02), but this correlation did not persist after correction for multiple comparisons.
Opioid Neurotransmission During Sustained Sadness
Consistent with a previous report (
19), significant regional increases in BP
ND were observed in the comparison group during the sadness state compared to the neutral state. These were localized in the pregenual and subgenual anterior cingulate (x, y, z coordinates in mm=3, 31, 4; cluster size=862 mm
3; z=5.91; average change 20%), the right nucleus accumbens/ventral pallidum (x, y, z=3, –3, –5; cluster size=300 mm
3; z=6.29; change 7%), the left nucleus accumbens (x, y, z=–16, 3, 1; cluster size=444 mm
3; z=4.74; change 12%), and the right hypothalamus (x, y, z=3, –3, –5; cluster size=300 mm
3; z=6.29; change 15%). This directionality (increase in BP
ND) is thought to reflect an acute reduction in endogenous opioid system tone (deactivation of neurotransmission) (
19,
20).
The borderline personality disorder sample showed increases in BPND that were localized in the left nucleus accumbens (x, y, z=–8, 10, –11; cluster size=220 mm3; z=4.10; change 5%), the hypothalamus (x, y, z=–1, –3, –10; cluster size=506 mm3; z=4.42; change 5%), and an area of brainstem in the approximate location of the periaqueductal gray and subjacent dorsal raphe (x, y, z=4, –21, –10; cluster size=418 mm3; z=3.35; change 12%); the latter was not significant after correction for multiple comparisons.
Increases in negative affect during sadness were correlated with reductions in opioid neurotransmission in the hypothalamus area in the comparison group (r=0.57, p=0.03) but not in the borderline personality disorder group. No significant correlations were observed between regional opioid system deactivation and scores on the Barratt Impulsiveness Scale, the NEO-PI-R neuroticism subscale, or the Dissociative Experiences Scale.
In contrast to a previous report (
19), healthy comparison subjects showed additional evidence of endogenous opioid system activation (reductions in BP
ND) in the left anterior thalamus (x, y, z=–11, 11, 18; cluster=13; z=5.16; 13% change), the left medial thalamus (x, y, z=–4, –10, 5; cluster=2066; z=5.65; 12% change), and the right hippocampus (x, y, z=29, –11, –24; cluster=632; z=5.16, p=0.013; 20% change). More widespread areas with a different regional distribution of endogenous opioid system activation during sadness were seen in the borderline personality disorder group. These included the left inferior temporal cortex (x, y, z=–24, 13, –33; cluster=64; z=5.43; 20% change), the left posterior thalamus (x, y, z=–15, –25, 17; cluster=113; z=4.87; 12% change), the right ventral pallidum (x, y, z=14, –1, -11; cluster=1481; z=5.98; 11% change), and the left amygdala (x, y, z=–21, 2, –19; cluster=81; z=4.56; 9% change). After correcting for multiple comparisons, no significant correlations between PANAS subscales or total scores and regional endogenous opioid system activation were observed for either group. No other correlations between endogenous opioid activation and other scales survived correction for multiple comparisons.
Group Differences in Response to Sustained Sadness Induction
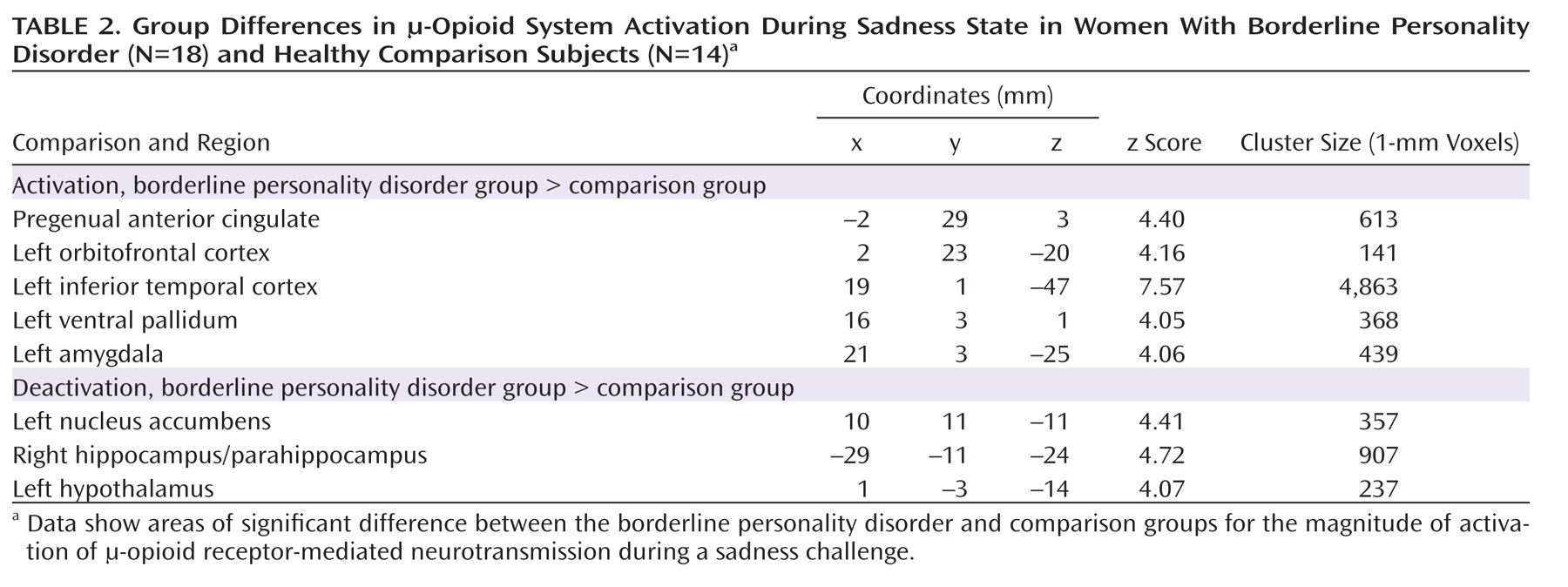
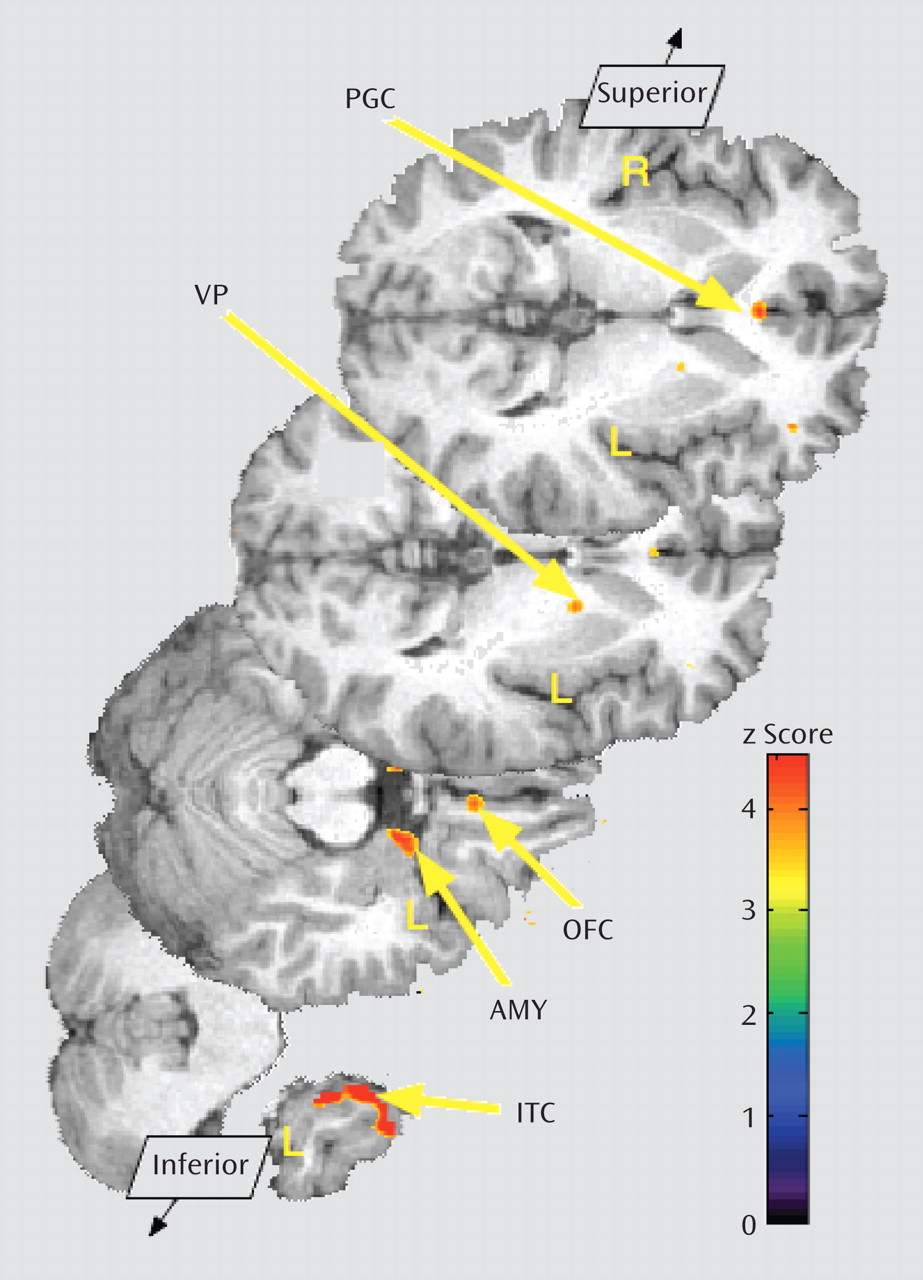
Borderline personality disorder patients demonstrated greater endogenous opioid system activation relative to comparison subjects during sadness in the pregenual anterior cingulate, left orbitofrontal cortex, left ventral pallidum, left amygdala, and left inferior temporal cortex (
Table 2;
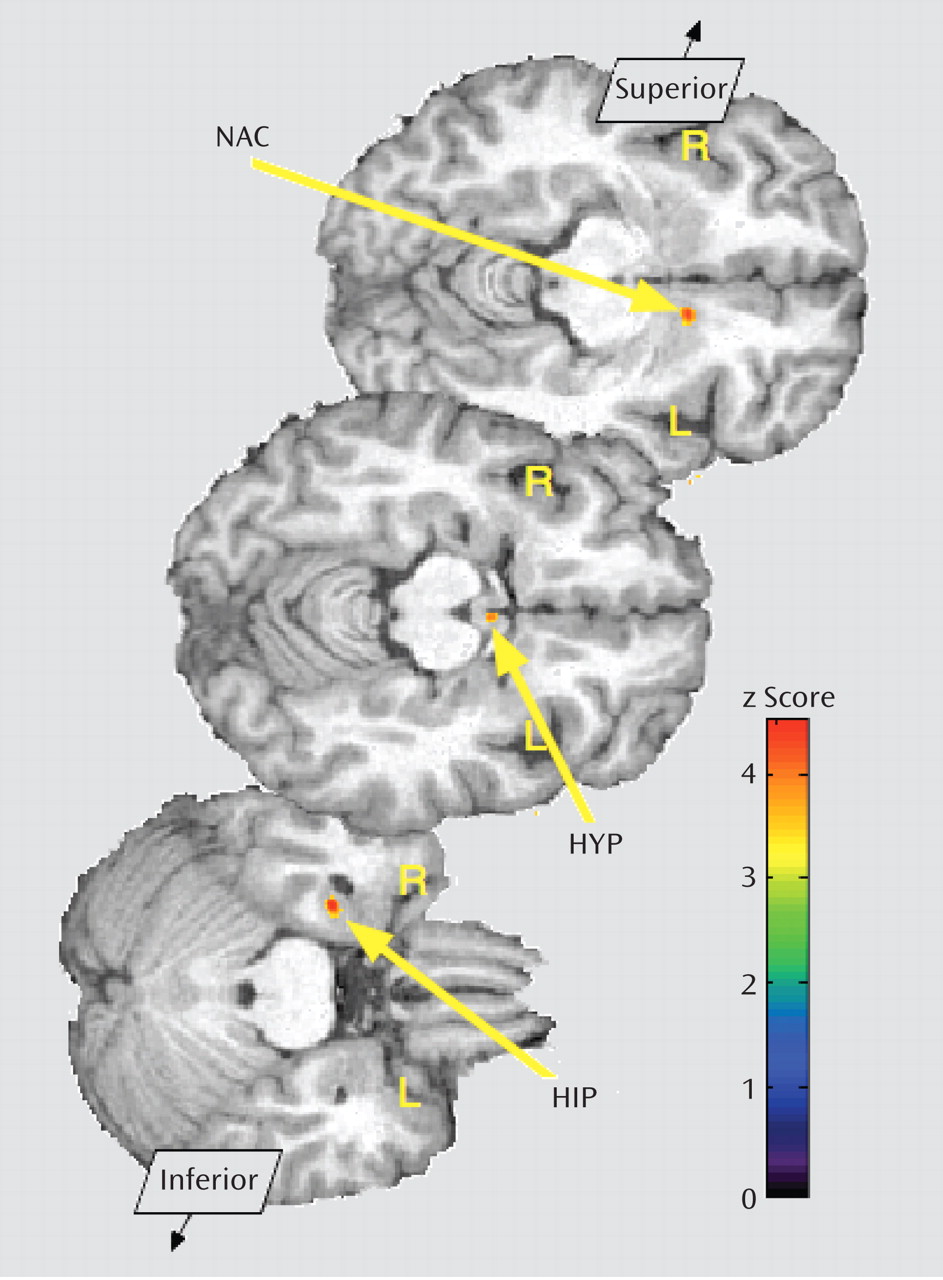
Figure 3). In the opposite direction, significantly greater regional deactivation of opioid neurotransmission in borderline personality disorder patients relative to comparison subjects occurred in the left nucleus accumbens and the right hippocampus/parahippocampus (Table 2;
Figure 4).
Discussion
We believe this is the first report addressing μ-opioid receptor-mediated neurotransmission alterations directly measured with molecular imaging techniques in borderline personality disorder. We found significant differences in baseline regional μ-opioid receptor availability in vivo, as well as in this neurotransmitter system's response to an emotional challenge in borderline personality disorder patients relative to matched comparison subjects. The endogenous opioid system and μ-receptors are thought to be involved in borderline personality disorder because of increases in pain thresholds and dissociative phenomena that are reversed by opioid antagonists (
23,
35). In animal models, the endogenous opioid system has been implicated in bond-forming and affiliative responses; emotion and stress regulation, including stress-induced analgesia; and impulsive-like behavior (
18). In humans, regional endogenous opioid system activation has been associated with suppression of both sensory and affective qualities of stressors and with trait impulsivity (
19,
22,
24,
36). Regional deactivation of the endogenous opioid system has been related to hyperalgesic responses and increases in negative affect during stress (
19,
29,
36). The general consensus is that activation of the μ-opioid system typically has a suppressive effect during emotional or physical challenges that threaten organism homeostasis.
Baseline μ-Opioid Receptors
We observed greater baseline (neutral state) μ-opioid receptor availability in borderline personality disorder patients relative to comparison subjects in cortical (orbitofrontal cortex) and subcortical (caudate, nucleus accumbens, amygdala) regions. We hypothesized that greater receptor availability may occur because of lower baseline endogenous neurotransmitter tone. Increases in BP
ND may reflect increases in actual receptor protein or increases in the proportion of high-affinity receptors (i.e., coupled with transduction mechanisms) preferentially labeled by agonist radiotracers (
37). Frequently these processes occur simultaneously and are interpreted as receptor up-regulation compensatory to lower endogenous opioid system tone. In rodent models, regional μ-opioid receptor protein and mRNA up-regulation have been described in response to social and experimental stress (
38) and as a consequence of opioid peptide depletion (
39). The opposite effect was observed in the posterior thalamus in humans, with reductions in μ-opioid BP
ND in borderline personality disorder. This coincides in location and directionality with effects found in major depressive disorder, related to poor antidepressant response and hyperactivity of the hypothalamic-pituitary-adrenal axis (
19,
20).
Response of μ-Opioid Neurotransmission During Sustained Sadness
Relative to comparison subjects, patients with borderline personality disorder demonstrated greater activation of the endogenous opioid system in response to sustained sadness in the pregenual anterior cingulate, left orbitofrontal cortex, left ventral pallidum, and left amygdala. These highly interconnected brain regions are implicated in evaluation and behavioral responses to salient stimuli and in decision making in healthy volunteers. Orbitofrontal cortex lesions are associated with poor decision making and difficulties in switching cognitive strategies (
40). This region has extensive connections with the cingulate gyrus, nucleus accumbens, amygdala, hippocampus, and hypothalamus (
41), which together form networks implicated in assessing saliency, intensity, and valence of rewards and stressors and in regulating behavioral responses (
42,
43).
The left lateralization of many of the results is interesting. Left lateralization has been described during successful retrieval of emotional context in the amygdala and in frontotemporal networks (
44).
Mu-opioid receptors in the ventral pallidum have been implicated in encoding hedonic value of rewards (
45), regulation of midbrain dopaminergic inputs, and integration of amygdala and prefrontal cortex connections, affecting motivated behavior (
46). Activation of the endogenous opioid system in the orbitofrontal cortex, anterior cingulate, thalamus, nucleus accumbens, ventral pallidum, and amygdala has been shown to suppress both physical and emotional aspects of stressful challenges (
19,
22,
47). Consistent with those findings, we observed negative but nonsignificant correlations between endogenous opioid system activation in the left amygdala and Barratt Impulsiveness Scale scores in the borderline personality disorder group. There were also negative but nonsignificant correlations between left inferior temporal cortex activation and increases in negative affect during sadness, again suggesting that in borderline personality disorder the endogenous opioid system is involved in the suppression of emotional responses. This interpretation corresponds to reports of relief from aversive arousal during cutting and self-injury (
48) and increased pain thresholds in patients with borderline personality disorder (
23).
Patients with borderline personality disorder were also differentiated from comparison subjects by a relative deactivation of μ-opioid neurotransmission in the nucleus accumbens, the hippocampus/parahippocampus, and the hypothalamus. The nucleus accumbens is implicated in the assignment of salience to both positive and negative events (
42). The hippocampus and parahippocampus have roles in explicit memory encoding and retrieval and in autonomic nervous system and neuroendocrine regulation through hypothalamic connections (
49).
Brain regions where increases in neutral state μ-opioid receptor availability were observed largely overlapped with those in which greater endogenous opioid system activation was found during sadness induction. If increases in baseline receptor availability reflect lower basal levels of dynamic regulatory control of emotional states and stress by the typically suppressive μ-opioid system, the response of these circuits during recall of negative experiences would be consistent with exaggerated stress-like responses to emotional stimuli that were not observed in comparison subjects.
Greater μ-opioid availability and endogenous opioid release in response to painful stimuli appear to be associated to impulsivity traits in healthy volunteers in locations overlapping with our findings here (
24). Serotonergic mechanisms, long implicated in borderline personality disorder pathophysiology (
50,
51), along with dopaminergic systems, have been associated with various forms of impulsive behavior as well. Our findings are consonant with the concept that both borderline personality disorder and impulsivity are multifactorial, affecting relevant circuits and involving various neurotransmitter systems, including the endogenous opioid system (
24). For example, well-described neurotransmitter-neurotransmitter interactions reveal regulation of serotonergic and dopaminergic cell firing by μ-opioid receptors in raphe (
52) and ventral tegmental nuclei (
53), respectively.
This study was limited by using small numbers of study subjects and including only women. While we think the findings are intriguing, we must be cautious in assuming that they are readily generalizable.
Clinical Overview
Linehan et al. (
7) suggested that borderline personality disorder is characterized by a low threshold to becoming emotionally dysregulated, followed by a rise to high levels of emotional arousal, with a slow intensity decrement over time. Greater emotional lability and dysregulation may occur in patients with borderline personality disorder because they do not attribute the correct saliency to an emotional event or stimulus (greater nucleus accumbens deactivation). Once they overattribute an emotion to an event, they cannot shift away from that emotion or shift their emotional or interpersonal strategy (orbital frontal cortex overactivation), even when the strategy is not interpersonally productive. The stimulus may become encoded with much greater emotional intensity (amygdala-hippocampal effects) than it should have, leading to greater reactivity to future similar emotional events.
Overall, we have described initial evidence of regional alterations in the function of the endogenous opioid system and µ-opioid receptors in brain regions involved in emotion and stress processing, decision making, and pain and neuroendocrine regulation. Further investigation into interindividual variations of the µ-opioid system is warranted.
Acknowledgments
The authors acknowledge the contributions of the nuclear medicine technologists of the Positron Emission Tomography Center at University of Michigan (Jill M. Rothley, Edward J. McKenna, Andrew R. Weeden, Paul Kison, and Shayna Huber) to the conduct of the studies, and Sujata Guduri, M.H.S.A., for her role in participant recruitment and coordination of data collection.