Personality traits are often related to psychopathology (
1). Investigating the neural substrates of personality traits may inform etiological and pathophysiological models of psychiatric disorders. In the case of psychosis, attention has focused on the relationship between schizotypal personality traits and schizophrenia spectrum disorders. Schizotypal personality traits encompass a broad range of personality characteristics and experiences, including unusual perceptions and beliefs, social anxiety or withdrawal, and disorganized thoughts or behaviors (
2). These traits cluster into positive, negative, and disorganized factors that are conceptually similar to the symptom dimensions of schizophrenia (
2–
4). The expression of schizotypal traits ranges from benign odd perceptual experiences or beliefs to severe symptoms associated with significant psychosocial impairment and schizotypal personality disorder (
2). Premorbid personality in schizophrenia is marked by an excess of schizotypal traits, and schizotypal personality disorder is a risk factor for schizophrenia (
5–
7). Indicators of cerebral dysfunction observed in schizophrenia spectrum disorders, including cognitive impairment and sensory gating deficits, are correlated with schizotypal traits in psychiatrically healthy individuals, which further underscores the link between schizotypal traits and schizophrenia spectrum disorders (
8–
14).
The neural basis of individual differences in schizotypal personality traits is poorly understood. Dopamine signaling may be associated with normal variation in these traits, given that dopamine dysregulation is prominent in schizophrenia spectrum disorders. Positron emission tomography (PET) imaging with displaceable dopamine receptor ligands sensitive to endogenous dopamine levels has shown that patients with schizotypal personality disorder demonstrate exaggerated dopamine release in the striatum following
d-amphetamine challenge (
15). Schizophrenia patients also demonstrate increased
d-amphetamine-induced dopamine release in the striatum (
16,
17). Dopamine release is especially robust in schizophrenia patients who are experiencing an acute illness exacerbation and, in contrast to patients with schizotypal personality disorder, is correlated with a transient increase in positive psychotic symptoms (
16).
Determining the relationship between dopamine transmission and individual differences in schizotypal traits may further our understanding of dopamine dysregulation in schizophrenia spectrum disorders. While the findings in schizophrenia provide a compelling case for a state component to hyperdopaminergia, the evidence for a trait basis is less conclusive, given that it is based largely on findings from one study of schizotypal personality disorder that included relatively few patients (
15). Moreover, the extent to which dopamine signaling varies continuously with dimensional measures of schizotypy is unknown. Evidence that dopamine signaling is correlated with individual differences in schizotypy would lend considerable support to the hypothesis that there is a trait component to hyperdopaminergia and may further suggest that hyperdopaminergia is an endophenotype of schizophrenia spectrum disorders.
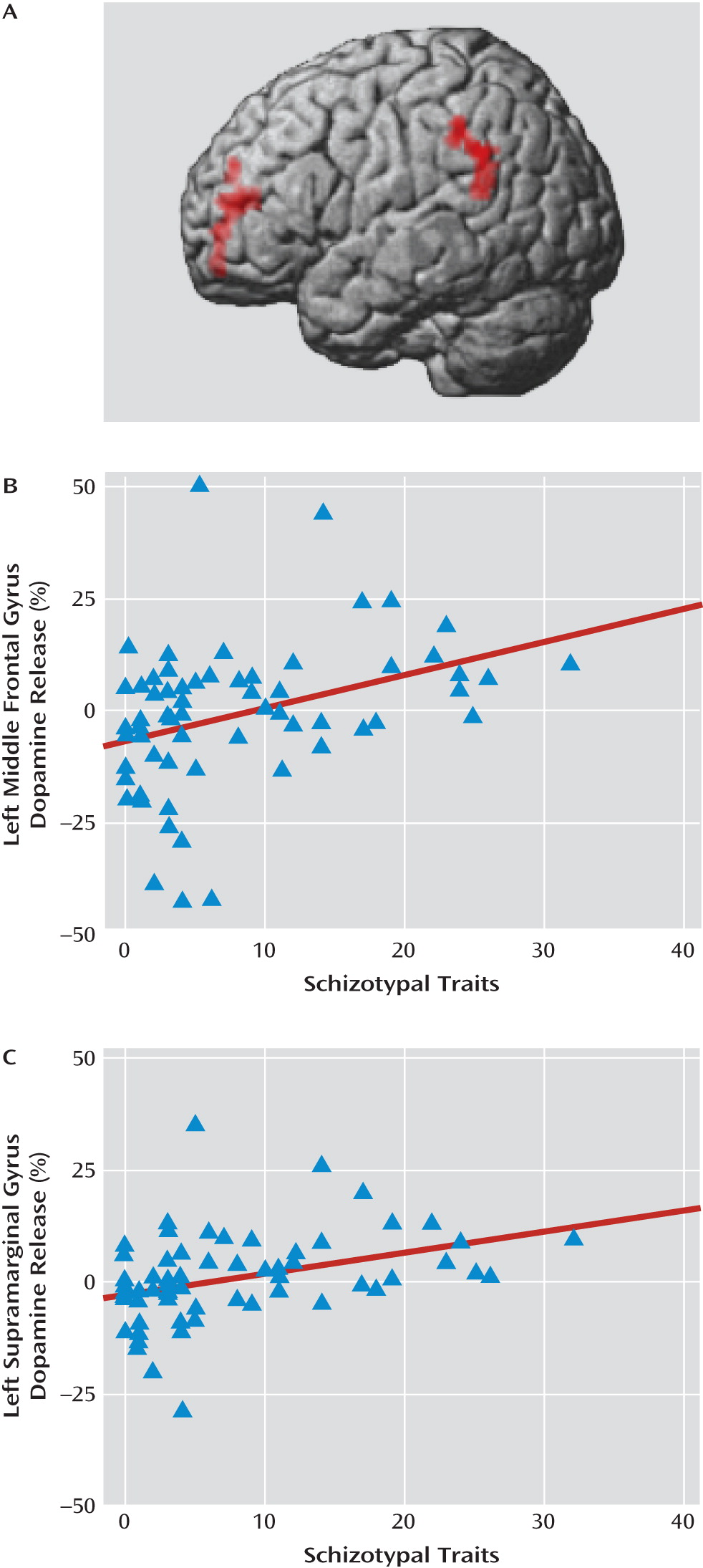
It is also unknown whether dopamine dysregulation in schizophrenia spectrum disorders includes extrastriatal brain regions. There are reasons to suspect that it may, given reports of elevated
l-dopa uptake in the amygdala and medial prefrontal cortex in schizophrenia (
18,
19) and findings from a recent meta-analysis showing that dopamine receptor occupancy by antipsychotics in the temporal cortex is strongly related to clinical efficacy (
20). Examining the relationship between schizotypal personality traits and extrastriatal dopamine transmission may provide testable hypotheses on the role of extrastriatal dopamine transmission in schizophrenia spectrum disorders.
Discussion
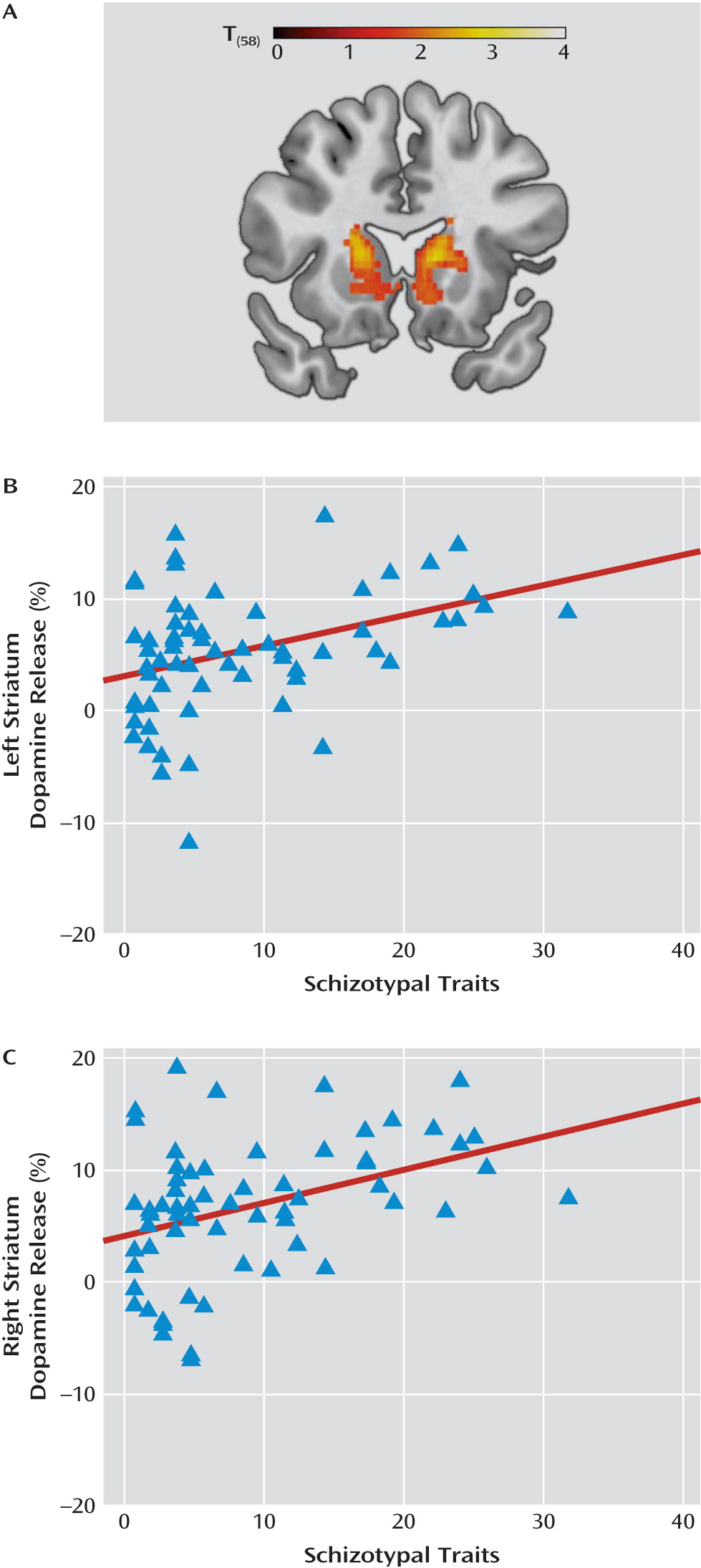
Dopamine release in striatal and extrastriatal brain regions is correlated with individual differences in schizotypal traits. Our findings suggest that the link between
d-amphetamine-induced dopamine release and schizophrenia spectrum disorders extends to normal variation in schizotypal personality traits. These results parallel associations previously reported between schizotypal traits and relative impairments in cognition and sensory gating in samples with similar Schizotypal Personality Questionnaire scores (
8–
14). Moreover, the correlations between dopamine release and schizotypal traits reported here are similar in magnitude to those reported from previous investigations of the association between psychometrically measured schizotypy and behavioral measures of cognition or sensory gating.
These findings may further our understanding of dopamine dysregulation in schizophrenia spectrum disorders. Extrapolating dopamine release based on the correlation we observed for the striatum region of interest, we obtain predicted dopamine release values of 9%–13% for Schizotypal Personality Questionnaire scores between 30 and 40 (the range reported in patients with schizophrenia and schizotypal personality disorder [
13,
33–
35 ]). This is similar to the 10%–12% increase observed in schizotypal personality disorder and remitted schizophrenia patients, but substantially less than the 20%–22% increase reported in acutely ill schizophrenia patients (
15). Although caution is warranted when making comparisons between studies that used different radioligands, slightly different
d-amphetamine doses, and different delivery routes (intravenous versus oral), the similarity in mean striatal dopamine release between the control sample (N=57) reported by Abi-Dargham et al. (
15) (7%–7.5%) and the present study (∼6%) supports the validity of this comparison. Thus, our findings support the hypothesis that the modest elevation in dopamine release observed in schizotypal personality disorder and remitted schizophrenia is a stable trait indicator related to schizotypy, while the robust increases reported in acutely ill schizophrenia patients is probably a state component superimposed on a trait-wise elevation in dopamine transmission (
15).
Identifying the neural basis of individual differences in personality traits associated with psychiatric illnesses is similar to imaging genetics approaches examining relationships between brain structure/function and putative psychiatric disorder risk genes in healthy individuals. By providing further support for a trait basis for dopamine dysfunction, our findings suggest that
d-amphetamine-induced dopamine release may represent an endophenotype of schizophrenia spectrum disorders. Evidence that unaffected relatives of patients with schizophrenia demonstrate elevated presynaptic dopamine synthesis capacity in the striatum also implicates hyperdopaminergia as an endophenotype for schizophrenia (
36). Identification of gene variants associated with psychostimulant-induced dopamine release may provide clues to the genetic basis of schizophrenia spectrum disorders.
Imaging studies of dopamine release in clinical studies have been limited to the striatum; however, there are reasons to suspect that hyperdopaminergia in schizophrenia spectrum disorders extends beyond the striatum (
20). Our findings showing a relationship between schizotypal traits and dopamine release in prefrontal regions may at first glance appear to contradict the cortical hypodopaminergia hypothesis of schizophrenia (
37). However, the evidence supporting cortical hypodopaminergia in schizophrenia is indirect and inconsistent. PET studies of cortical dopamine D
1 receptors in schizophrenia have reported increased, decreased, and unaltered levels (
38–
40). Moreover, inferences about dopamine function based on differences in receptor levels observed between patients and comparison subjects under normal physiological conditions may not generalize to stimulant challenge. We find little evidence that baseline BP
ND is associated with dopamine release in our data. Thus, it is possible that hypodopaminergia inferred from differences in baseline BP
ND may be unrelated to amphetamine-induced dopamine release, or may actually co-occur with stimulant-induced hyperdopaminergia. Studies of extrastriatal amphetamine-induced dopamine release in schizophrenia spectrum disorders are clearly warranted.
Multimodal imaging may inform the relationship between schizotypy, especially disorganized traits, brain function, and dopamine signaling. The face validity of disorganized factor questions suggests that they may be related to subtle limitations in executive cognitive functions. Individual differences in disorganized schizotypal traits are correlated with executive function, and abnormal prefrontal cortical functioning during task performance is correlated with disorganized symptoms in schizophrenia patients (
9,
33,
41–
43). The prefrontal cortex influences dopamine function directly by altering midbrain dopamine cell firing and indirectly through presynaptic innervation of striatal dopamine terminals (
44,
45). Consequently, alterations in prefrontal cortical function may alter the response of both cortical and subcortical dopamine systems to amphetamine challenge.
This study has several limitations. Our findings require replication in a sample with a broader range of schizotypal traits in order to better characterize the relationship between specific facets of schizotypy and dopamine release. Also, because we did not rule out axis II psychopathology during screening, it is possible that some participants met criteria for schizotypal personality disorder; this is unlikely, however, given the range of Schizotypal Personality Questionnaire scores in this sample. It is also unlikely that our results are related to other dimensions of psychopathology, such as anxiety and depression, given that neither is associated with dopamine release (
46,
47). Interview-based measures may be more sensitive to schizotypal traits than self-report questionnaires, which raises the possibility that different results might have been obtained had we used interview-based methods (
48). Finally, combining study subjects across amphetamine administration protocols and PET scanners is not ideal. However, our findings were largely unchanged when analyses were restricted to the placebo-controlled cohort, and we did not find any differences in dopamine release between scanners.