Cognitive impairments, such as deficits in working memory, are among the most disabling and difficult to treat features of schizophrenia (
1,
2) and appear to be linked to impairments in the circuitry of the prefrontal cortex (
3). For example, markers of gamma-aminobutyric acid (GABA) neurotransmission are altered in subpopulations of prefrontal cortex GABA neurons (
4), and evidence of disturbed glutamate neurotransmission, such as N-methyl-D-aspartic acid (NMDA) receptor hypo-function (
5), has also been reported in the illness. Thus, understanding the mechanisms that might contribute to disturbed GABA and glutamate neurotransmission is essential for developing novel treatments for cognitive impairments in schizophrenia.
Group I metabotropic glutamate receptors (mGluR1α, mGluR5) play an important role in regulating GABA and glutamate neurotransmission in the cortex. For example, activation of group I mGluRs initiates an intracellular signaling cascade that leads to suppression of GABA release (
6–10) and long-term potentiation of NMDA receptor function (
11). Interestingly, one study reported higher protein levels of mGluR1α, but not mGluR5, in the prefrontal cortex of elderly schizophrenia subjects (
12), which may contribute to altered GABA and glutamate neurotrans-mission in schizophrenia. However, it remains unclear whether group I mGluR alterations are generalizable to most subjects with schizophrenia, including younger individuals with the disorder, and whether alterations in group I mGluR protein levels are attributable to differences in gene expression levels.
Activation of group I mGluRs initiates a G protein signaling pathway, which is inhibited by regulator of G protein signaling 4 (RGS4) (
13). RGS4 functions as a GTPase-activating protein that increases the hydrolysis of guanosine triphosphate (GTP) and reduces the duration of activity of several different G protein-coupled receptors, including group I mGluRs (
13). RGS4 has been previously identified as a gene of interest in schizophrenia because of genetic association studies (
14) and reports of lower expression levels in the prefrontal cortex in two relatively small cohorts of subjects with schizophrenia (
15,
16). However, it is unclear whether lower RGS4 mRNA levels are commonly found in schizophrenia and whether these lower levels, which may affect the duration of intracellular signaling from group I mGluRs, are present in the same schizophrenia subjects who have higher group I mGluR levels.
The endogenous cannabinoid system is also critically involved in regulating GABA and glutamate neurotrans-mission in the cortex. For example, the endocannabinoid 2-arachidonoylglycerol is synthesized by diacylglycerol lipase (
17) in pyramidal neurons (
18,
19) and then travels retrogradely to stimulate cannabinoid 1 receptors located in nearby axon terminals from certain inhibitory neurons (
20) and, to a lesser extent, other pyramidal neurons (
21), leading to a suppression of GABA and gluta-mate release (
21,
22). 2-Arachidonoylglycerol activity is then terminated through metabolism by monoglyceride lipase (
23). Interestingly, the endocannabinoid system also appears to be the primary mediator of the aforementioned suppressive effects of group I mGluRs on GABA release. For example, activation of group I mGluRs, but not other classes of mGluRs, leads to increased synthesis of 2-arachidonoylglycerol (
24) and suppression of GABA release (
6–10) in a manner that is dependent upon the cannabinoid 1 receptor (
7,
9,
10). We recently reported lower cannabinoid 1 receptor mRNA and protein levels in the prefrontal cortex in individuals with schizophrenia (
25). However, fully understanding the pathophysiological state of the endocannabinoid system as well as the downstream effects on GABA and glutamate neuro-transmission in schizophrenia also requires knowledge of whether the level of 2-arachidonoylglycerol is altered in the disorder. Levels of 2-arachidonoylglycerol cannot be directly assessed in postmortem human brain tissue (
26) but can be inferred, in part, by measures of gene products that regulate 2-arachidonoylglycerol synthesis (diacylglycerol lipase) and degradation (monoglyceride lipase). To our knowledge, no studies have yet reported the status of endocannabinoid ligands in schizophrenia.
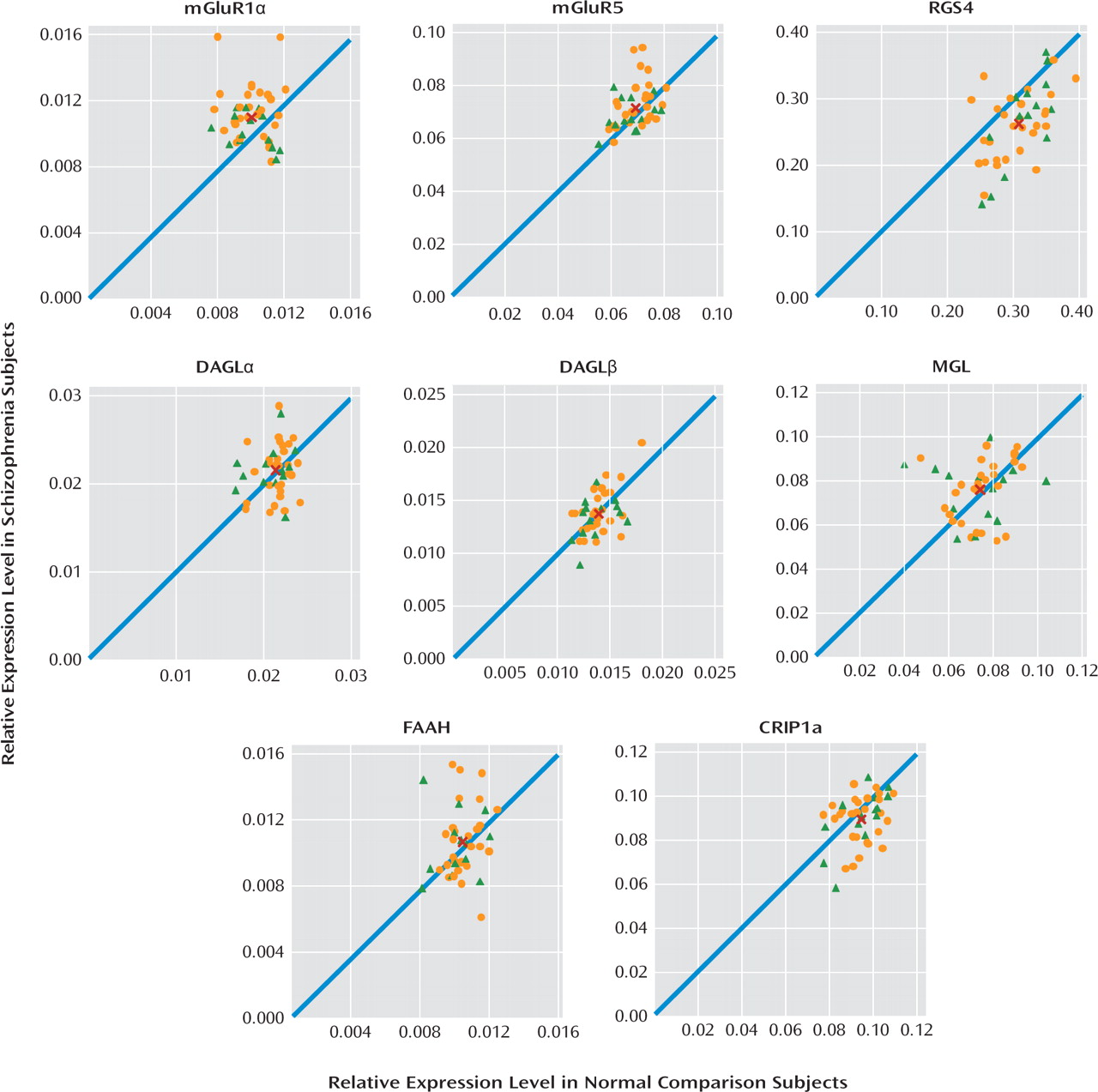
Therefore, in the present study, we sought to evaluate the status of several important regulators of GABA and glutamate neurotransmission in the prefrontal cortex by examining the transcript levels of mGluR1α, mGluR5, RGS4, and endocannabinoid synthesizing (diacylglycerol lipase) and metabolizing (monoglyceride lipase) enzymes in a relatively large cohort of schizophrenia subjects (N=42). In schizophrenia subjects, we found elevated mRNA levels for mGluR1α, but not mGluR5, and lower RGS4 mRNA levels, but transcript levels for diacylglycerol and monoglyceride lipase did not differ between subject groups. Higher mGluR1α and lower RGS4 mRNA levels may have an important impact on GABA and glutamate neurotransmission in schizophrenia.
Discussion
In this study, we found that mRNA expression levels for mGluR1α, but not mGluR5, were significantly higher in the prefrontal cortex in schizophrenia subjects. Consistent with prior reports (
15,
16), we also found lower mRNA levels for RGS4, which reduces the duration of G protein-mediated intracellular signaling from mGluR1α activation (
13). Lower RGS4 mRNA levels were commonly found in the same schizophrenia subjects who had higher mGluR1α mRNA levels. In contrast, no differences were found in mRNA levels for the synthesizing and metabolizing enzymes for 2-arachidonoylglycerol, diacylglycerol lipase (both α and β isoforms), and monoglyceride lipase, respectively, or for fatty acid amide hydrolase, the metabolizing enzyme of the other cortical endocannabinoid, anandamide, in schizophrenia. Taken together, these data suggest that altered mGluR1α and RGS4 mRNA levels may be common in schizophrenia and may represent a disturbed “molecular hub” that has an important effect on multiple components of neuronal communication in the prefrontal cortex.
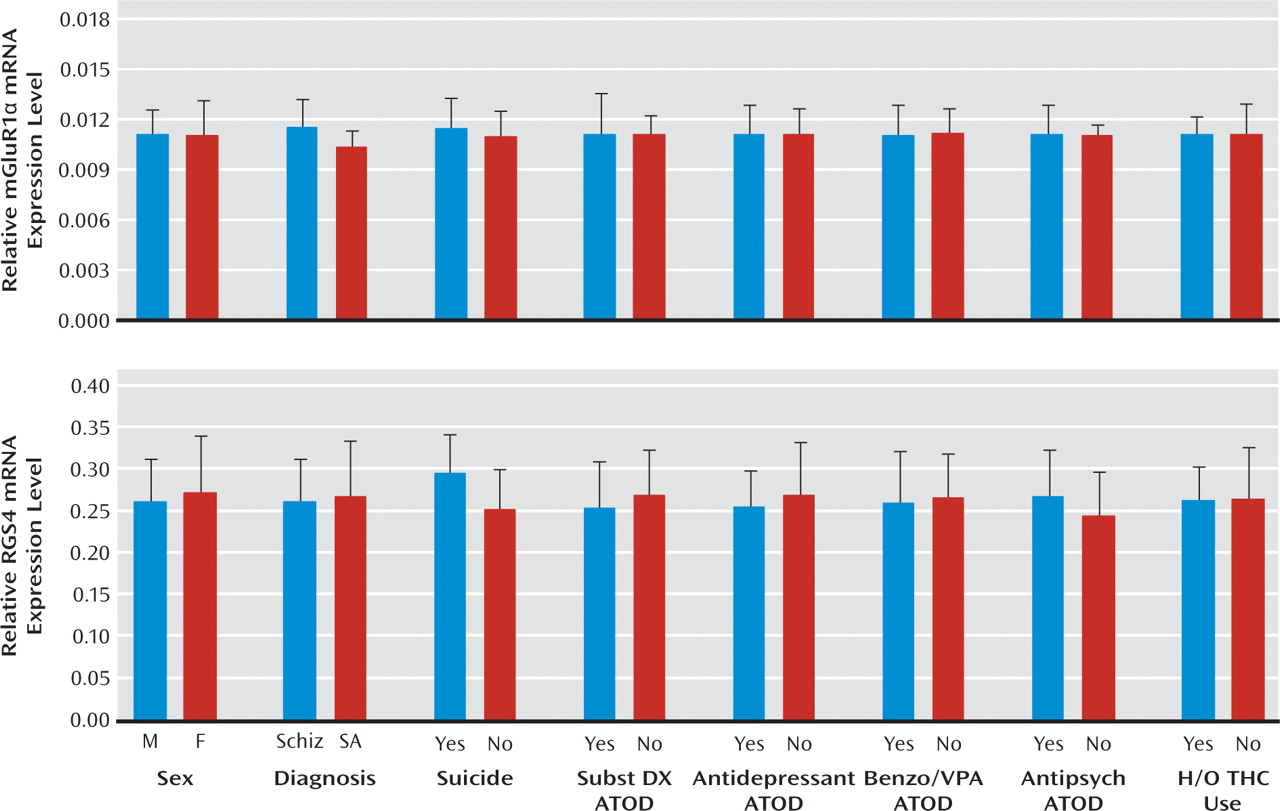
Increased mGluR1α mRNA levels appear to be specific to the disease process of schizophrenia, or at least not attributable to factors frequently associated with the illness. For example, treatment with antipsychotic medications did not appear to affect mGluR1α mRNA levels, since these levels were similar in schizophrenia subjects who were and were not receiving medications at the time of death. In addition, in contrast to our findings in schizophrenia subjects, mGluR1α mRNA levels were slightly lower in antipsychotic-exposed monkeys, although these differences did not achieve statistical significance. Furthermore, differences in sex; suicide as a cause of death; diagnosis of substance abuse or dependence current at the time of death; the use of antidepressants, benzodiazepines, or sodium valproate at the time of death; or a history of cannabis use did not appear to affect mGluR1α levels in schizophrenia subjects. Interestingly, mGluR1α mRNA levels and mGluR5 mRNA levels appeared to be higher in schizophrenia subjects than in schizoaffective disorder subjects. However, no other markers showed a difference between schizophrenia and schizoaffective disorder subjects, including the cannabinoid 1 receptor (
25).
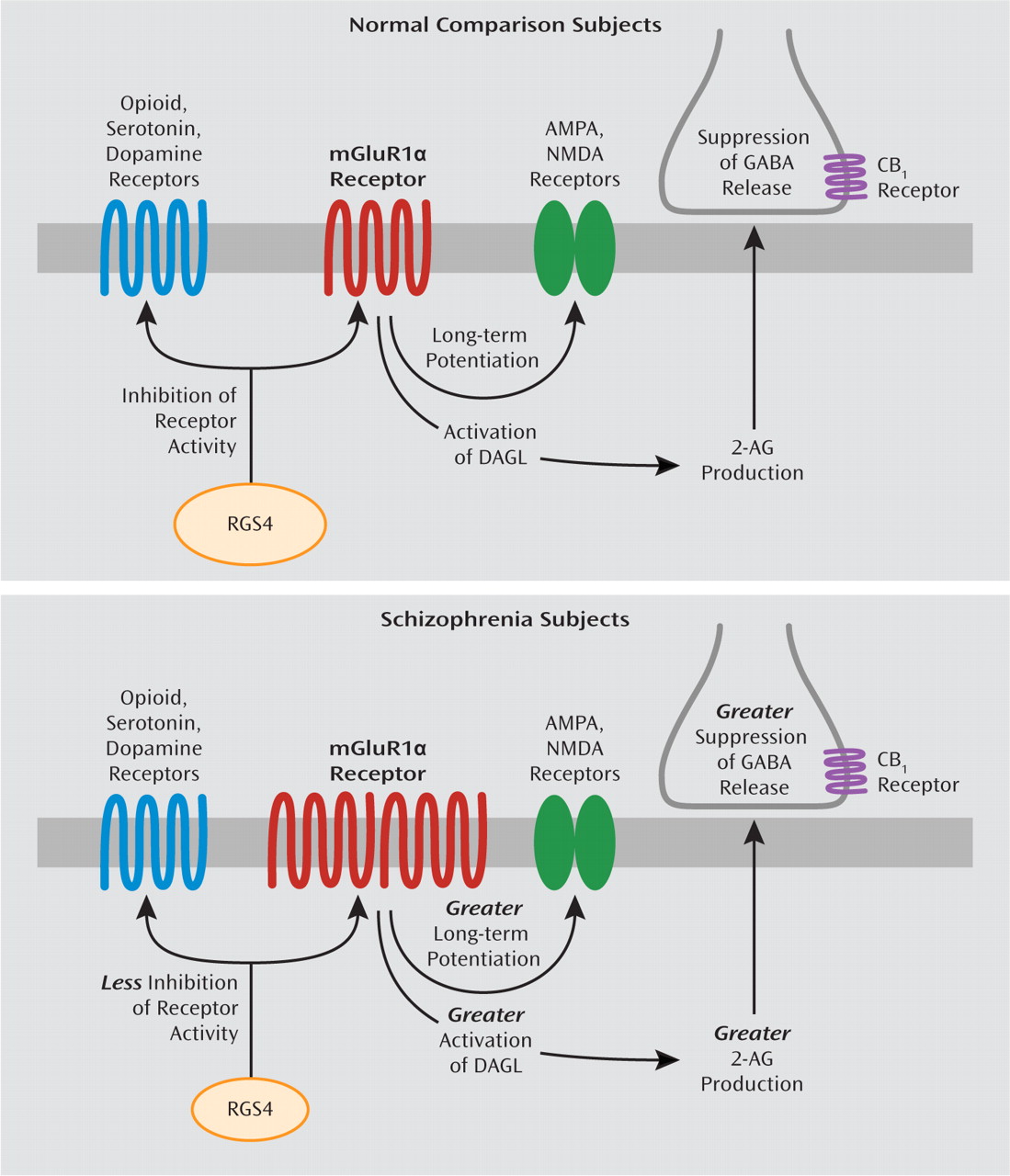
Several lines of evidence suggest that the capacity for mGluR1α activation may be increased in the prefrontal cortex in schizophrenia. First, our findings of elevated mGluR1α mRNA levels parallel a previous report of higher mGluR1α protein levels in the prefrontal cortex in schizophrenia (
12), suggesting that higher levels of mGluR1α mRNA expression may lead to translation of more protein, although this hypothesis needs to be directly tested in the same subjects. Second, RGS4 reduces the duration of intracellular signaling from mGluR1α (
13). Consistent with evidence that RGS4 also regulates G protein coupling for multiple other receptors (
36–39), RGS4 and mGluR1α mRNA levels were not correlated in individual subjects, and differences in RGS4 and mGluR1α mRNA levels between schizophrenia and matched normal comparison subjects were not correlated in subject pairs. However, out of the 42 subject pairs, 27 pairs showed both higher mGluR1α and lower RGS4 mRNA levels in the schizophrenia subject relative to the matched normal comparison subject. Taken together, these data suggest that higher mGluR1α and lower RGS4 mRNA levels are generally found in the same schizophrenia subjects and, if accompanied by changes in levels of the corresponding proteins, may lead to a greater capacity for, and a longer duration of intracellular signaling from, mGluR1α activation in the prefrontal cortex in schizophrenia (
Figure 4).
As previously mentioned, alterations in RGS4 mRNA levels in schizophrenia may have additional effects beyond modifying the intracellular signaling efficacy of mGluR1α. First, RGS4 is a GTPase-activating protein that reduces the duration of activity of several different G protein-coupled receptors in addition to mGluRs, including dopamine, serotonin, and opioid receptors (
36–39). Thus, lower RGS4 mRNA levels, if also present at the protein level (
16), may enhance the functioning of an array of receptors in schizophrenia (
15) (
Figure 4). Second, some, although not all (
40), case-control and family-based association studies have identified single nucleotide polymorphisms (SNPs) of the RGS4 gene as susceptibility factors for schizophrenia, although different alleles of these SNPs have been associated with schizophrenia in different subject populations (
14,
41). Some of the risk haplotypes for RGS4 are also associated with smaller volume of the prefrontal cortex (
42) and altered activation of the prefrontal cortex during working memory tasks (
43) in schizophrenia subjects. Thus, alterations in RGS4 at the genomic and transcript level may have a broad effect on diverse neurotransmitter systems and on functional properties of the prefrontal cortex in schizophrenia.
Higher mRNA levels for mGluR1α and lower mRNA levels for RGS4, if accompanied by altered levels of the corresponding proteins (
12,
16), are consistent with a higher capacity for mGluR1α-mediated neurotransmission in schizophrenia, which may have a diverse and complicated effect on multiple components of neural transmission in the disorder. For example, immunoreactivity for mGluR1α and RGS4 is found in both pyramidal neurons and GABA neurons in the primate prefrontal cortex (
44,
45). An important limitation of our study is that a tissue-level analysis of mRNA levels does not allow a determination of a potential cell-type specificity of higher mGluR1α and lower RGS4 mRNA levels in schizophrenia, and thus follow-up studies are needed to determine whether altered levels of mGluR1α and RGS4 are predominantly found in pyramidal neurons or GABA neurons or both. With this caveat in mind, we will conclude by focusing on the potential downstream effect of greater mGluR1α-mediated neurotransmission on the NMDA receptor, endocannabinoid, and GABA systems in schizophrenia. For example, activation of group I mGluRs results in long-term potentiation of NMDA receptor function (
11), and NMDA receptor function appears to be impaired in schizophrenia (
5). Thus, these lines of evidence invite speculation that in schizophrenia, greater mGluR1α activity may have a partially compensatory effect for NMDA receptor hypofunction. In addition, activation of group I mGluRs has been reported to increase synthesis of 2-arachidonoylglycerol (
24) and suppress GABA release (
6–10). mGluR1α-mediated activation of 2-arachidonoylglycerol synthesis has been reported to occur through a G protein-coupled mechanism that leads to increased levels of substrate (i.e., diacylglycerol) for diacylglycerol lipase (
46). Therefore, while mRNA levels for the synthesizing and metabolizing enzymes for 2-arachidonoylglycerol are not altered in schizophrenia, higher mGluR1α activity may increase the substrate-dependent activity of diacylglycerol lipase, even in the absence of changes in its enzyme level. Thus, higher mGluR1α-mediated activation of 2-arachidonoylglycerol synthesis may further suppress GABA release, which may worsen GABA-ergic deficits in schizophrenia. Higher mGluR1α mRNA levels and lower RGS4 mRNA levels may also affect additional components of neural transmission, including AMPA, dopamine, serotonin, and opioid receptors (
11,
36–39) (
Figure 4). Taken together, these data suggest that altered mGluR1α and RGS4 mRNA levels may represent a disturbed molecular hub that has an important effect on multiple neurotransmitter systems in schizophrenia. Additional studies characterizing the cell-type specificity of transcript and protein level alterations in mGluR1α and RGS4 may provide greater insight into their downstream effects on prefrontal cortex circuitry, and potentially their relationship to cognitive impairments, in schizophrenia.