Stimulants such as methylphenidate and amphetamines are the most commonly used psychotropic medications in children, being widely prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) (
1,
2). Stimulant use increased steeply in the United States during the 1990s, rising from a point prevalence of 0.6% in 1987 to 2.7% in 1997 (
3). In the years that followed, use appeared to stabilize around an estimated rate of 2.9% in 2002 (
4). Reports since then, however, suggest that the use of these medications may have continued to rise (
5).
According to the National Health Interview Survey, approximately 9% of children ages 6–17 had ever received a diagnosis of ADHD in 2006, with a wide geographical variation across the United States (
6). These rates are consistent with the 9% lifetime prevalence of ADHD diagnosis by age 18 in the National Comorbidity Survey–Adolescent Supplement database, which was collected between 2001 and 2004 (
7). Based on the National Survey of Children's Health, the percentage of children ages 4–17 who had ever received a diagnosis of ADHD increased from 7.8% to 9.5% between 2003 and 2007, with a 5.5% annual rate of increase (
8). It is estimated that about two-thirds of the children diagnosed with ADHD receive pharmacological treatment (
8).
In the past decade, a number of new formulations of methylphenidate and amphetamines have been introduced into clinical practice, including controlled release oral and transdermal preparations (
9). As the market for ADHD medications has expanded, concerns have been raised about the possible misuse and abuse of stimulants, especially because the increase in ADHD diagnoses has been most marked in adolescents (
4,
10,
11).
Trends based on short periods of time can be imprecise and misleading. In this study, we used data from an ongoing nationally representative annual survey to describe the overall trend of pediatric stimulant use from 1996 to 2008, with special attention to utilization by age, sex, race/ethnicity, and geographic region.
Method
Data Sources
The data were drawn from the data files for years 1996–2008 of the Medical Expenditure Panel Survey (MEPS). The MEPS is a nationally representative household survey of health care use and costs conducted by the Agency for Healthcare Research and Quality and has been used extensively to track trends in mental health treatment in the United States (
3,
4,
12,
13). The MEPS uses an overlapping panel design, combining two panels to produce estimates for each calendar year (with the exception of 1996, when the survey began). Households for each panel are interviewed five times over a 2-year period. The sample for each panel is drawn from the sample of all households responding to the National Health Interview Survey in the year prior to the panel start date in the MEPS. Overall response rates for the MEPS for 1996–2008 ranged from 56.9% to 70.2%. Our analytic sample includes all individuals age 18 and younger in each year. Final annual sample sizes varied with the number of households sampled each year in the MEPS and ranged from 6,595 to 11,713. The MEPS sample is poststratified to the Current Population Survey and is representative of the civilian noninstitutionalized population in each year.
Data on prescription drug use in the MEPS were collected both directly from households and from a follow-back survey of all pharmacies reported by the household, for which a signed permission form was obtained. Detailed information obtained from responding pharmacies (85% response rate), including National Drug Code, drug name, strength, and form for each drug fill, were matched back to the prescription drug fills reported by the household. We defined stimulants to include the following compounds in various formulations: methylphenidate, dexmethylphenidate, pemoline, amphetamines, and dextroamphetamine.
The data for 1996 through 2008 match previously reported estimates from the MEPS and allowed us to assess trends across a full 12-year period (1996–2008) (
4). We also replicated previously reported estimates on stimulant use for the population age 18 and younger using data from the 1987 National Medical Expenditure Survey (NMES), the predecessor to the MEPS, allowing us to estimate trends over a 21-year period.
Data Analysis
We computed national estimates of the annual use of stimulants for the U.S. civilian noninstitutionalized population of children age 18 and younger for calendar years 1996 through 2008 using MEPS data and replicated estimates for 1987 using NMES data. We also computed changes between 1996 and 2008 for the following population subgroups: age, sex, race/ethnicity, family income relative to the federal poverty line, census region, metropolitan statistical areas (MSAs) versus non-MSAs, health insurance coverage, and impairment as measured by the Columbia Impairment Scale (
14).
Table 1 provides the distribution of these subgroups across the population age 18 and young for 1996, 2002, and 2008.
MEPS sampling weights, which adjust for the stratified sample design and for nonresponse, are used throughout the analyses. We used two-tailed t tests, computed with these weights and accounting for the complex sample design and correlation across individuals, to assess changes in stimulant use between 1996 and 2008, between 1996 and 2002, and between 2002 and 2008. Logistical regression analysis was used to further examine the sociodemographic correlates of stimulant use in children ages 5–17 for whom the Columbia Impairment Scale measure was available, pooling the years 2006 through 2008 to increase power to detect differences.
We have 90% power to detect an average 0.20 percentage point change per year over 5-year intervals at an alpha level of 0.05. This is a smaller average increase than was observed between 1987 and 1997 (0.26 percentage points). We report all statistical tests based on annual estimates, but we also recomputed all tests pooling data for consecutive years to test the robustness of the results.
All statistical analyses and tests were performed using Stata/MP, version 11.1 (StataCorp, College Station, Tex.).
Results
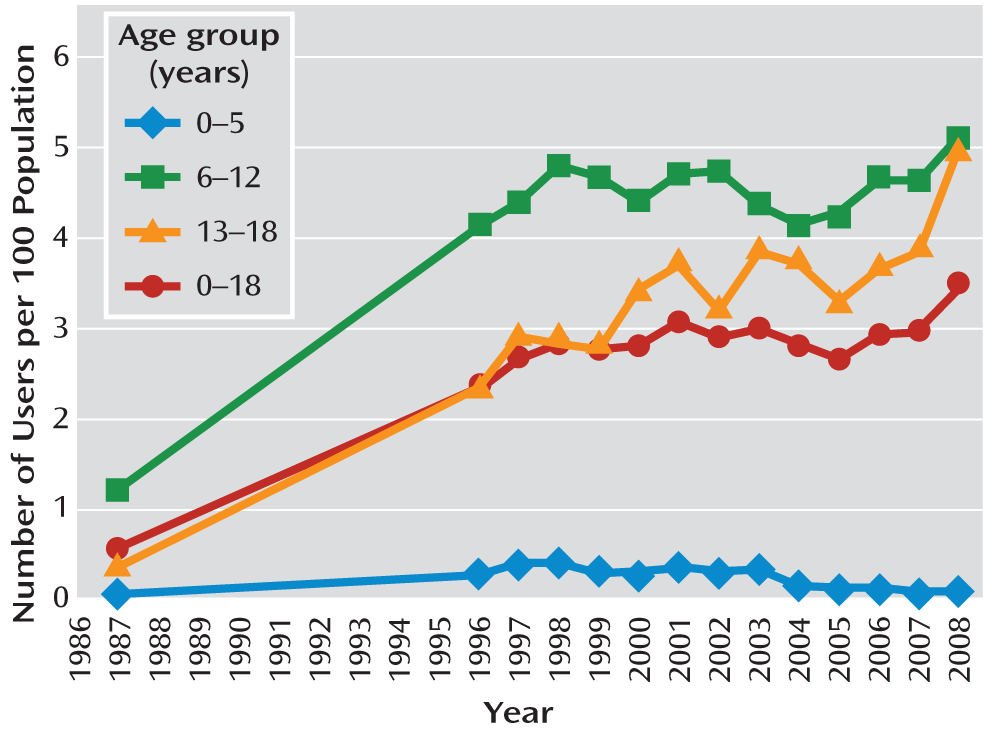
An estimated 2.8 million (95% confidence interval [CI]=2.3–3.2) children (individuals age 18 and younger) received stimulant medication in 2008. The utilization rate was 3.5% (95% CI=3.0–4.1) in 2008, representing a statistically significant increase from 1996 (2.4%; 95% CI=1.8–2.9) (
Table 2 and
Figure 1). This translates into a compound annual growth rate of 3.4% for the period 1996–2008. This growth was substantially slower than the annual growth rate of 17.0% between 1987 and 1996.
Across all years of observation (1987, 1996–2008), stimulant use was highest in 6- to 12-year-olds. In this age group, use was 4.2% (95% CI=3.2–5.2) in 1996 and 5.1% (95% CI=4.1–6.1) in 2008, with no statistically significant change. Over the same period, use in 13- to 18-year-olds increased at an annual rate of 6.5%, from 2.3% (95% CI=1.5–3.1) in 1996 to 5.0% (95% CI=3.9–6.1) in 2008. Use in children under age 6 remained low (0.1% from 2004 onward), with a statistically significant decrease from the period before 2004 to the period afterward (t=3.71, p<0.001).
Use was more than three times greater in boys than in girls in 2008, thus returning to the same male-to-female ratio as in 1996 (
Table 3). The difference between boys and girls had narrowed between 1996 and 2002 as use increased in girls at a faster rate than in boys, but then widened again between 2002 and 2008 as use increased in boys but not in girls.
Use remained consistently greater in non-Hispanic white children (4.4% in 2008) than in African American (3.0%) or Hispanic (2.1%) children (
Tables 3 and
4).
Use remained similar across all income groups in the period 1996–2008 as each group experienced similar patterns of increase (
Table 3). Children without insurance, however, continued to have lower utilization (1.3% in 2008) than those with private (3.4%) or public (4.3%) insurance. Among the insured, children with public coverage tended to have greater utilization than those with private coverage (odds ratio=1.36, t=2.14, p=0.016).
Significant differences in use were found across regions of the United States (
Table 3). Rates of utilization increased substantially in the Northeast (from 2.7% in 2002 to 4.6% in 2008, with much of the spike coming in the last year). In contrast, the West maintained a consistently lower rate of utilization, with no increase in recent years (1.2% in 1996, 2.2% in 2002, and 1.6% in 2008).
Although stimulants constitute the first-line pharmacological treatment of ADHD, they are not the only medications available for children with this disorder. We also estimated the pediatric use of other commonly prescribed ADHD medications using the MEPS database for recent years. For 2007–2008, the mean rate of clonidine and guanfacine use combined was 0.3% (95% CI=0.2–0.4) overall, 0.6% (95% CI=0.3–0.8) in 6- to 12-year-olds, and 0.3% (95% CI=0.1–0.5) in 13- to 18-year-olds. Most of those who used clonidine or guanfacine (80.2%, 95% CI=67.1–93.3) also used stimulants during the previous 12 months, although not necessarily concurrently. The mean rate of atomoxetine use for 2007–2008 was 0.6% (95% CI=0.4–0.7) overall, 0.8% (95% CI=0.5–1.1) in 6- to 12-year-olds, and 0.8% (95% CI=0.5–1.0) in 13- to 18-year-olds. One-third of those who used atomoxetine (32.7%; 95% CI=18.9–46.5) also used stimulants during the year. The rate of atomoxetine use was 0.9% (95% CI=0.7–1.0) for 2004–2005, which was significantly higher than for 2007–2008 (t=2.44, p=0.015).
Discussion
These data, which are based on a nationally representative annual survey, show that the pediatric use of stimulants has continued to grow at the same pace since the mid-1990s. This relatively slow growth is in sharp contrast to the rapid increase that occurred between the mid-1980s and the mid-1990s. However, the overall growth rate for children age 18 and younger masks important changes that have occurred within different age groups. Stimulant use in 13- to 18-year-olds increased in the period 2002–2008, with use rates converging with those of 6- to 12-year-olds. In contrast, use in preschoolers has declined.
Stimulant use in children from racial and ethnic minority groups has increased, although the rate remains lower than in non-Hispanic white children. In addition, while stimulant use is greater in girls now than it was 10 years ago, boys retain a threefold higher utilization rate, which is consistent with the higher prevalence of ADHD in boys (
6–
8). Finally, significant differences by region persist, with the West having significantly lower use.
If one compares these estimated rates of utilization of stimulant medication with the estimated prevalence of the ADHD diagnosis in the community (
6–
8), it appears that most children diagnosed with ADHD are not treated with stimulants. This may not be unexpected, since about half of those diagnosed present with only mild symptoms (
6–
8) and since other treatments, including psychosocial interventions and nonstimulant medications, are available. In the absence of biological markers for ADHD, the validity of community diagnoses is uncertain, and the MEPS database does not allow diagnostic validity to be examined, even though it represents an ecologically valid estimate of the prevalence of community diagnoses. In any case, the data show that stimulant use is higher in children with significant functional impairment as indicated by the Columbia Impairment Scale (
Tables 3 and
4), further supporting the notion that these medications tend to be prescribed for the more severe forms of the disorder.
The significant increase in stimulant utilization in racial and ethnic minorities and low-income families indicates an increased recognition of ADHD and acceptance of its pharmacological treatment by the groups for whom disparities in mental health services have traditionally existed. But the persistence of differences in use among racial and ethnic groups also indicates that social and cultural factors continue to play a significant role in ADHD treatment utilization. Parents of Hispanic and African American children are less likely to report ADHD than parents of white children, and these differences are not accounted for by health or socioeconomic variables, such as birth weight, income, and insurance coverage (
15).
The continuous, steep increase in stimulant utilization in adolescents likely reflects the recent realization that ADHD tends to persist in puberty, causing significant functional impairment (
16,
17). Data from the U.K. show a steep increase in ADHD medication prescribing for youths over the years 1999–2006, thus indicating that this phenomenon is not limited to the United States (
18). The increasing use in this age group does little to assuage the concerns raised about the potential for misuse and diversion of these medications (
11).
An age group in which use has remained extremely low and has actually declined over the period of 1996–2008 is that of preschoolers (
Table 2). In early 2000, much concern was raised about use of stimulants in young children (
19). Subsequently, a controlled clinical trial showed that methylphenidate is effective in preschoolers with ADHD but also causes more adverse effects in this group than in school-age children (
20). Our data indicate that in clinical practice, stimulants are seldom used in children under age 6, and the trend has been toward even lower use.
The significant differences we observed in stimulant use across regions of the United States are consistent with previous reports (
4,
21) and indicate a substantial variability in the approach to ADHD, which likely reflects differences in treatment preferences that deserve further inquiry. Differences in health care organization and delivery in the West compared with the rest of the United States may account for some of the observed discrepancy. However, the lower use of stimulant medication in the West does not seem to be paralleled by a lower pediatric use of other psychiatric medications, such as antidepressants (
22).
Several methodological limitations must be taken into account when interpreting these data. First, self-report surveys such as the MEPS rely on the respondents' ability and willingness to accurately recall information. Recall and reporting biases could result in underreporting and hence underestimates of use. While the MEPS is designed to make nationally representative estimates using probability-based sampling, the adjustments made for nonresponse may not completely eliminate the potential for nonresponse bias. Second, the MEPS does not include sufficient information for determining the validity of the reported ADHD diagnoses, although the validity of the MEPS data are supported by their consistency with data on drug expenditures from other sources (
23). A third limitation is the lack of detail and statistical power in the database regarding nonstimulant medications or the use of combined medications in children, a practice that has become increasingly common (
24). However, based on available data, it appears that the proportion of atomoxetine users is small and has decreased, while most children using clonidine or guanfacine also receive stimulants.