The recent advent of array-based genome-wide technologies has led to long-awaited progress in our understanding of the genetic causes of psychiatric disorders (
1). Developments have included the identification of a range of common and rare variants that contribute to disease susceptibility. A recent study found genome-wide significant association between bipolar disorder and rs1064395, which is a common variant in the 3′ untranslated region of the neurocan (
NCAN) gene (p=2.14×10
−9). This combined genome-wide association study (GWAS) and replication study involved a total of 8,441 patients and 35,362 controls (
2). The
NCAN finding is one of four genome-wide significant association findings for common variants reported for bipolar disorder to date, the others being ankyrin 3 (
ANK3) (
3), the α subunit of the
l-type voltage-dependent calcium channel (
CACNA1C), and
ODZ4, a human homologue of the
Drosophila pair-rule gene
ten-m (odz) (
4). These variants confer a small effect on disease susceptibility, and since the genetic contribution to bipolar disorder is largely polygenic (
5), many more common variants will be identified in future studies. However, each variant has the potential to open up new avenues into causal biological pathways and thus warrants further investigation.
Discussion
The results of our reverse phenotyping approach in a combined bipolar disorder/schizophrenia/major depression sample suggest that the NCAN risk allele plays a crucial role in the etiology of the mania dimension. Our attempt to refine the mania dimension revealed that the overactivity subdimension of mania drives the association between the mania dimension and the risk allele.
The fact that the association with phenotype dimension was observed across diagnostic categories is consistent with recent research, which has challenged the validity of the current classification systems. Although identification of disease-associated risk genes is based primarily on the use of categorical diagnostic systems, this process allows researchers to define phenotype dimensions that are primarily affected by a specific gene, as well as to investigate whether such an effect is present across diagnostic boundaries or is specific to a particular disease (
8,
23). These results have an impact on etiological concepts of disease and may thus have important consequences for diagnostic classification.
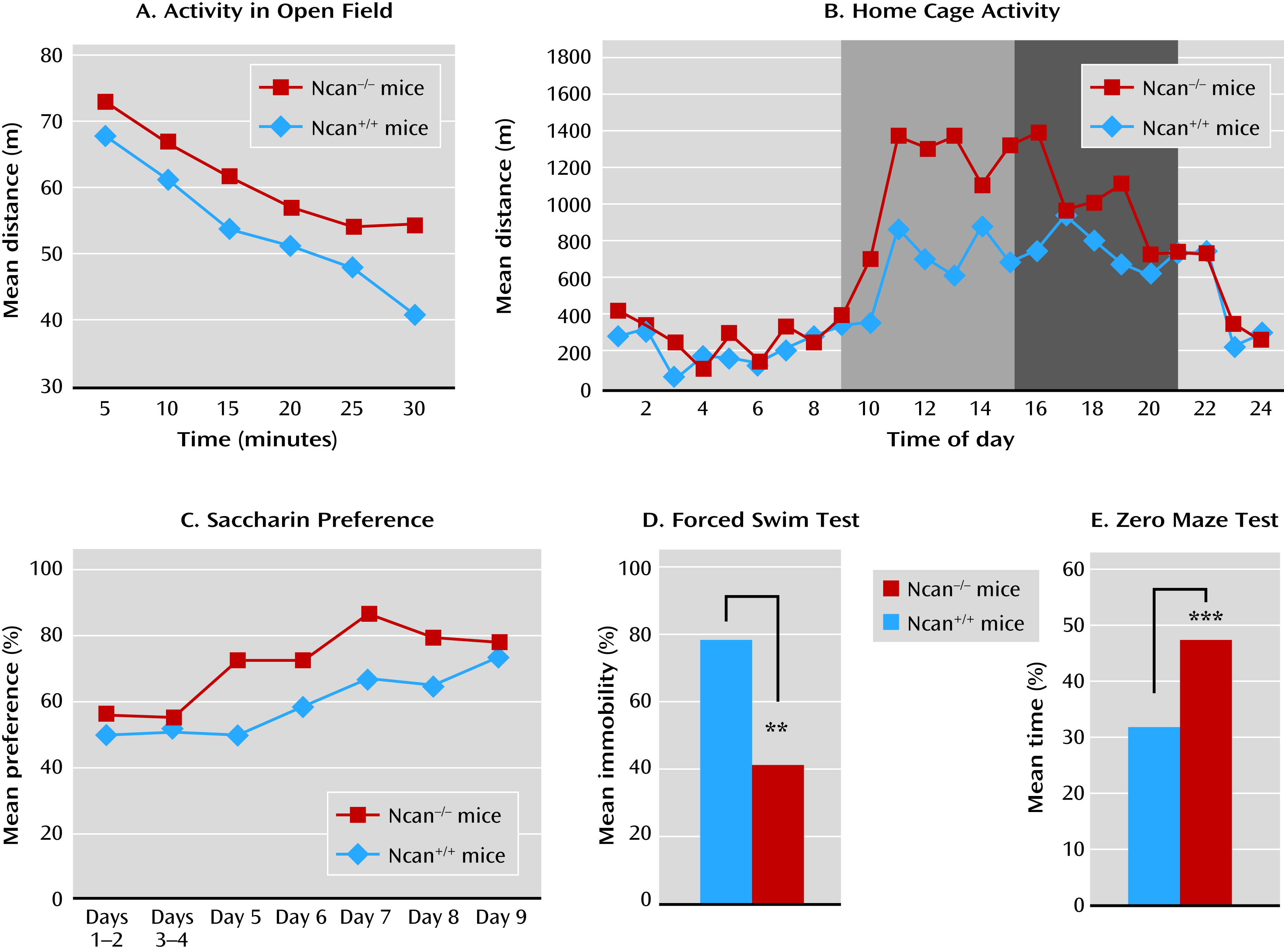
In the present study, Ncan−/− mice showed behaviors resembling those observed in the human mania phenotype. Ncan−/− mice displayed increased activity in their home cage as well as within the context of other behavioral paradigms, such as the open field, the Porsolt forced swim test, and the marble burying test.
Ncan
−/− mice displayed lower levels of anxiety-related behavior in both the zero maze and the elevated plus maze. This may model the greater risk-taking behavior of mania patients (
24). Interestingly, lithium treatment reduced risk-taking behavior not only in Ncan
−/− mice, but also in Ncan
+/+ mice.
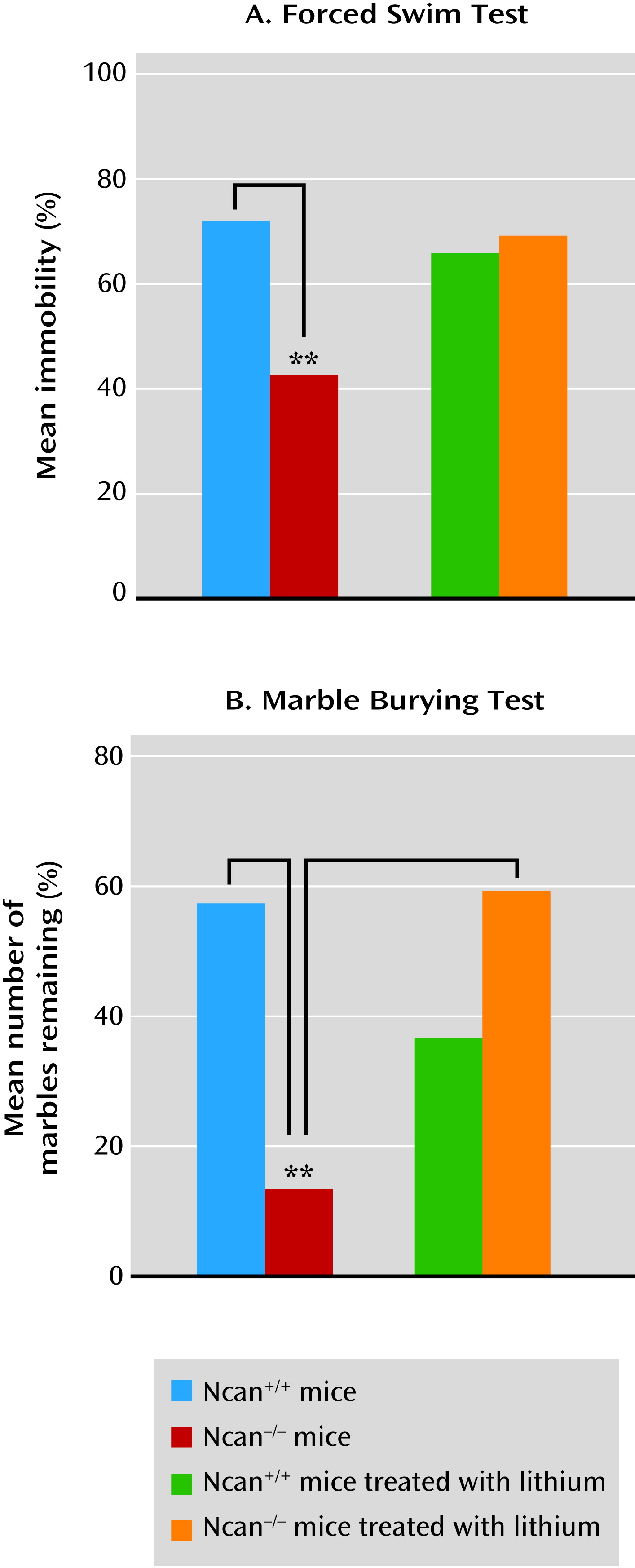
The Porsolt forced swim test is a widely used behavioral despair model with high predictive validity for antidepressant medications (
25). Thus, immobility time (behavioral despair) is decreased by all medications that have antidepressant efficacy in humans. However, the test may also be useful in the evaluation of enhanced vigor as a mania-related symptom (
26). Sodium valproate, another medication used for the treatment of mania, has been shown to increase immobility time in a Black Swiss mouse strain with a low immobility score. Our study provides strong support for this finding, as it demonstrated that lithium increased immobility time in Ncan
−/− mice (which also have a low Porsolt forced swim test immobility score) to the level observed in Ncan
+/+ mice.
Mice and other rodents have a natural tendency to bury objects introduced into their cage. Burying marbles has been interpreted as a defensive behavior toward novel, potentially harmful objects, since anxiolytic medications decrease both its extent and duration. A recent study exploring the contribution of components such as novelty-induced anxiety and innate digging behavior to the marble burying phenomenon (
27) questioned the specificity of marble burying as an indicator of anxiety. Defensive burying was not correlated with other anxiety-like traits but was strongly related to explorative/repetitive digging. Similarly, we observed no correlation in Ncan
−/− mice between the marble burying test and the response to the elevated plus maze. Therefore, the increased marble burying behavior observed in Ncan
−/− mice may reflect a component of excessive mania-like behavior rather than an anxiety-related response to the novelty of the marbles. Although the hyperactivity of this strain could result in an increase in marble burying behavior, the fact that lithium administration reduced marble burying without reducing the motor activity in the Ncan
−/− mice renders this explanation less probable.
Reduced preference for sweet solutions is frequently used as an indicator of depression-related anhedonia, as it is sensitive to antidepressant treatment (
28). In contrast, a greater preference for palatable sweet solutions (as observed in Ncan
−/− mice) is thought to indicate a hyperhedonic state and has been associated with euphoria and increased reward-seeking behavior in patients with mania (
26).
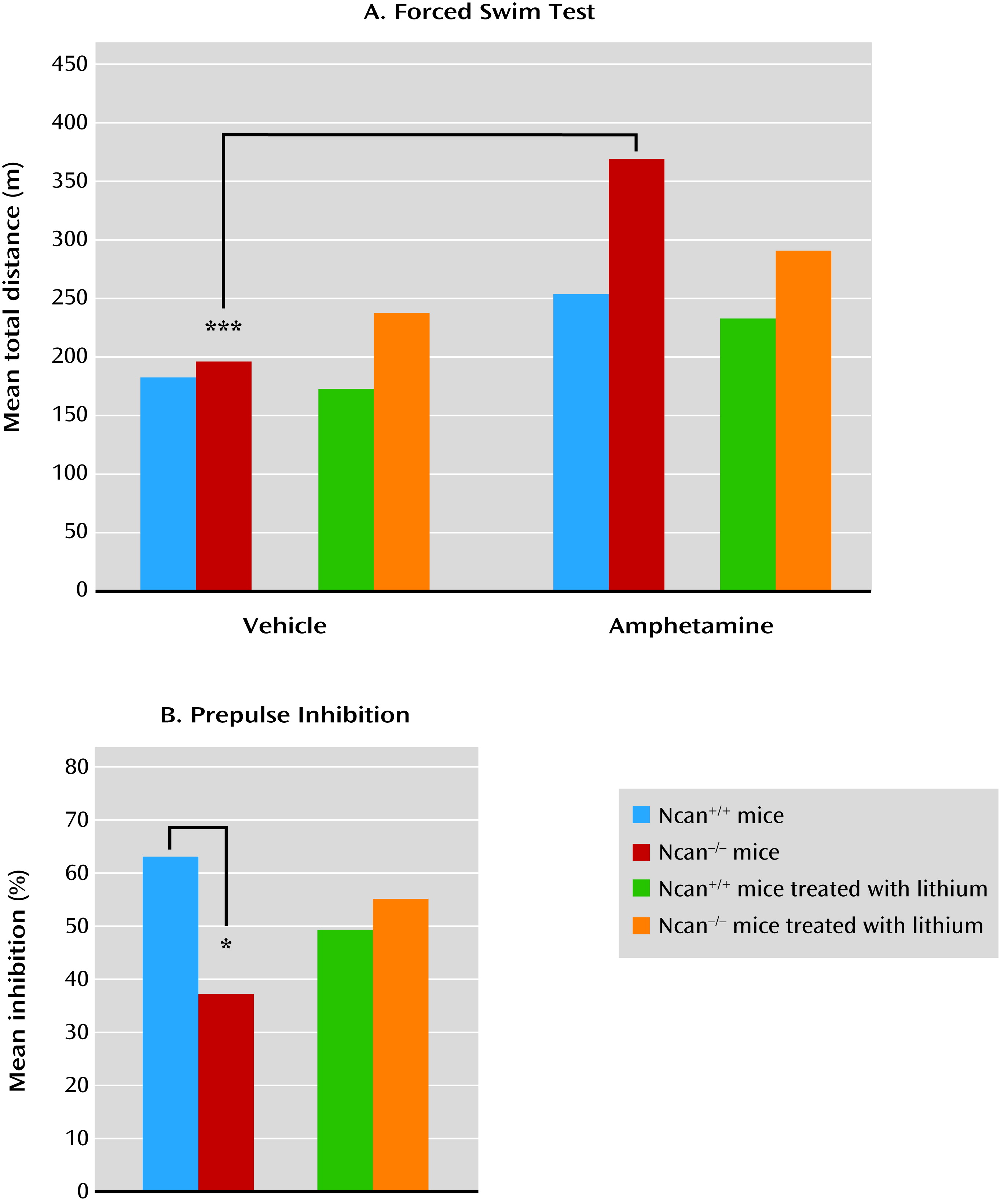
Although the psychotic symptoms observed in mania and schizophrenia cannot be modeled directly in mice, we can evaluate behaviors that are driven by neuronal circuits involved in the development of psychotic symptoms. For example, it is possible to measure sensitivity to psychotomimetic drugs using locomotor activity as a behavioral readout, or neurophysiological features such as sensorimotor gating (
29). In the present study, amphetamine was used as a psychotomimetic drug (
30). Ncan
−/− mice displayed a higher sensitivity to amphetamine, as reflected in an increase in locomotor activity. Lithium reduces psychostimulant-induced hyperactivity. In Ncan
−/− mice, lithium also normalized the amphetamine response. After the administration of lithium, Ncan
−/− mice no longer displayed a significant increase in locomotor activity in response to low-dose amphetamine treatment, as with Ncan
+/+ animals.
Ncan
−/− mice also showed deficient sensorimotor gating, as measured by prepulse inhibition of the acoustic startle response. This deficit has been reported in several studies of psychiatric patients, including those with mania (
31) or schizophrenia (
32). This deficit was also reversed by lithium treatment in Ncan
−/− mice.
Few genetic animal models with a mania-related phenotype have been reported to date (
17). Reported models include knockouts for GluR6 (
22), Clock (
20), Erk1 (
33), and Bcl-2 (
21) as well as glycogen synthase kinase 3-β (Gsk-3β) overexpressing transgenic mice (
34). A comparison between Ncan knockouts and these mouse models is provided in Table S4 in the
online data supplement. The behavioral phenotype of Ncan
−/− mice shows similarities with these mouse models, such as increased risk-taking behavior (GluR6 and Clock); greater activity, including home cage activity (NGluR6); reduced immobility in the Porsolt forced swim test (GluR6, Clock, Erk1, Gsk-3β); increased sucrose hedonia (Clock, Erk1, Bcl-2); and increased locomotor response to amphetamine (GluR6, Erk1, Bcl-2). These animal models enable investigation of the contribution of specific genes to specific aspects of disease pathology with well-defined and quantifiable measures (
7). Although an individual behavior can be affected by a number of gene mutations, the co-occurrence of these behavioral phenotypes suggests that the investigated genes have a specific involvement in the pathophysiological changes underlying mania. Notably, in all of these models (with the exception of Gsk-3β, which was not tested), a response to lithium or sodium valproate was observed.
The precise mechanism through which
NCAN influences illness risk remains unclear. As a component of the extracellular matrix of the CNS,
NCAN may exert its influence through the various important roles of the extracellular matrix in the control of key cellular events, such as adhesion, migration, proliferation, differentiation, survival, axon outgrowth, learning, and memory (
35,
36). Studies in humans and animals indicate that
NCAN plays a crucial role during early brain development and, to a lesser extent, postnatally (
2,
37). Therefore, it is possible that the observed behavioral changes result from deficits in the development of the central nervous system. This renders examination of its effect on psychopathology later in life difficult. However, postmortem studies have revealed a lower expression of
NCAN in Brodmann’s areas 46/10 in patients with bipolar disorder and major depression compared with controls (see
www.stanleygenomics.org). These areas map to the ventral lateral and ventral medial aspects of the prefrontal cortex and have been linked to higher-order emotion regulatory processes. Dysregulation of this region has been implicated in affective symptomatology (
38).