Since the study of the reproductive safety of antidepressants requires one to separate the effects of maternal depression from the effects of its pharmacological treatment, research examining these effects independently in children is warranted. Therefore, the purpose of this cohort study was to evaluate children’s neurocognitive and behavioral abilities following prenatal exposure to venlafaxine, SSRIs, or untreated maternal depression.
Method
Participants and Study Design
Participants were recruited from the prospectively collected data in the Motherisk program at the Hospital for Sick Children in Toronto. This database contains information about pregnant women who sought counseling on the pregnancy safety of medications, including antidepressants and nonteratogens. When they called Motherisk, oral consent was obtained for pregnancy follow-up and research participation. This procedure was approved by the research ethics board at the Hospital for Sick Children. Women who fulfilled our inclusion and exclusion criteria were selected.
Three groups of women with depression were selected: those who took venlafaxine during pregnancy (group 1), those who took SSRIs during pregnancy (group 2), and those who discontinued pharmacotherapy before conception (group 3). Group 4 consisted of nondepressed, healthy pregnant women who called Motherisk to inquire about nonteratogenic exposure (e.g., acetaminophen). From these callers, we selected the first ones who consented and had no psychiatric history.
Excluded were mothers exposed to polytherapy for depression or known teratogens (e.g., antiepileptic drugs), mothers with substance abuse (e.g., alcohol use disorders), mothers with other psychiatric conditions (e.g., schizophrenia), premature children (below 37 weeks of gestation), mothers and/or children with medical conditions unrelated to in utero exposure that may affect cognitive outcomes (e.g., postnatal head trauma, encephalitis), and mothers and/or children with inadequate English proficiency.
Procedures
At the time of the first contact with Motherisk during pregnancy, information about maternal medical, genetic, and obstetric histories was collected. Information about pregnancy course, medication changes, perinatal outcomes, and breast-feeding was ascertained through a routine Motherisk follow-up telephone call at 6 to 9 months after delivery. Information was also obtained regarding the severity, course, and treatment of depression at the time of the child’s assessment in order to identify potential confounding or predictive variables. Just before testing at the hospital, written consent statements were obtained from the parents and oral assent was obtained from the child.
Depressive episodes were defined according to DSM-IV criteria and were diagnosed by the woman’s psychiatrist. Only depressed women receiving pharmacotherapy before or during pregnancy were included. A 10-point visual analogue scale was used to assess the severity of depression in pregnancy and in each depressive episode after delivery. Maternal depression symptoms at the time of the child’s testing were assessed by using the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item self-report scale designed to measure depression symptoms (
6). It is scored from 0 to 60, with scores of 16 and above representing clinically significant depressive symptoms.
Anthropometric measurements, such as height, weight, and head circumference, were obtained for each child. A written report on the child’s health was also obtained from the child’s physician.
Intellectual and Behavioral Assessment
A psychometrist masked to group affiliation tested all children individually using a battery of age-appropriate standardized psychological tests.
The Wechsler Preschool and Primary Scale of Intelligence–Third Edition (
7) evaluated the children’s intelligence, providing three summary scores: full-scale IQ, which represents general intellectual functioning; verbal IQ, which represents verbal reasoning and comprehension; and performance IQ, which represents fluid reasoning, spatial processing, and visual-motor integration.
Behavioral profiles were assessed by using the Child Behavior Checklist (
8) and Conners’ Parent Rating Scale (
9), both completed by the mother. The Child Behavior Checklist provides scores on three broad factors: internalizing problems (depressed affect and withdrawn behaviors), externalizing problems (aggressive and delinquent behaviors), and total problems, which summarizes both. The Conners’ Parent Rating Scales global index and DSM-IV total symptom subscales were used to evaluate attention deficit hyperactivity disorder and other DSM-IV symptoms. Scales from both the Conners measure and Child Behavior Checklist are scored as T scores (mean=50, SD=10) with a higher score signifying more behavior problems.
The mother’s intelligence (assessed with the Wechsler Abbreviated Scale of Intelligence full-4 IQ [
10]), parental socioeconomic status (measured with the Hollingshead Four-Factor Index of Social Status [
11]), and household income were measured as potential confounders or predictors.
Statistical Analysis
Data analyses were conducted by using SPSS version 20 software (IBM, Armonk, N.Y.). Power calculation was performed by using the WINPEPI software to detect a full-scale IQ difference of eight points (clinically significant difference) between the groups exposed to venlafaxine and healthy women with α=0.05 (
http://www.brixtonhealth.com/pepi4windows.html).
Summary and descriptive statistics, as well as frequencies and normality of distribution, were assessed in order to apply parametric or nonparametric statistical tests.
One-way analysis of variance (ANOVA) was used to test for differences in continuous variables of interest among three or more groups; subsequent pairwise analysis was performed for multiple testing by using Tukey’s honestly significant difference test. We used t tests for two-group comparisons in continuous variables, and chi-square analyses were used to assess categorical outcomes. All analyses were two-tailed, with p≤0.05 considered significant.
The Pearson’s correlation coefficient was used to examine the relationships of cognitive and psychological outcomes to the extent of exposure to antidepressant medications and maternal depression. Variables that were deemed statistically significant or biologically plausible were chosen for subsequent hierarchical linear regression analyses. These included antidepressant dose, duration of antidepressant treatment during pregnancy (weeks), severity of depression during pregnancy and at the time of child testing, maternal IQ, and the child’s age and sex. These potential covariates were initially introduced into the model one by one and were evaluated in terms of statistical significance and effect on the main outcome. The model was performed in blocks to evaluate the strength of variables of interest as predictors.
The amount of missing data was minimal; there were no more than two missing data points for any variable. These were input with the group mean for statistical analysis.
Results
From 2001 to 2006, 608 women called Motherisk regarding antidepressant counseling for depression. Among them, 381 did not meet the inclusion criteria. In addition, 17 callers who took venlafaxine in pregnancy, 19 who took SSRIs in pregnancy, and 13 who went untreated during pregnancy were unable to be located or refused participation.
The intake forms of women who were lost to follow-up or declined to participate were analyzed. Their medical and psychiatric histories, medications, concomitant disorders, and demographic characteristics did not differ from those of the included cohort.
Sixty-two mother-child pairs in groups 1 and 2, 54 in group 3, and 62 in group 4 were tested. Group 2 included 11 women exposed to sertraline, 20 exposed to paroxetine, 15 exposed to citalopram, 15 exposed to fluoxetine, and one exposed to fluvoxamine. The venlafaxine defined daily doses ranged from 0.25 to 3.75, and the SSRI defined daily doses ranged from 0.40 to 4.00. Of the 124 women exposed to antidepressants, 81 were exposed throughout pregnancy, 21 in the first trimester only, four in the first and second, two in the second only, 11 in the second and third, and five in the third only. The median duration of antidepressant use during pregnancy was 30 weeks (range, 4–42 weeks).
Table 1 displays the descriptive statistics for all variables included in the final analysis. Maternal demographic characteristics and IQs were similar among all four groups. At testing, women from groups 1, 2, and 3 had experienced depression for similar numbers of years. However, women in group 3 received fewer years of pharmacotherapy than those in groups 1 and 2, although only the difference from group 1 was significant. There was no significant difference among the three groups with depression in terms of comorbidities such as anxiety, mania, and obsessive-compulsive symptoms (data not shown). Women from group 3 experienced more severe depression during pregnancy than those treated with venlafaxine or SSRIs, with a significant difference between groups 1 and 3. At the time of testing, all women with depression had similar scores on the CES-D.
A depressive episode in the first year following delivery was experienced by 79.6% of the women in group 3, 50.0% in group 1, 66.1% in group 2, and 3.2% in group 4 (p<0.001). Of the women in group 3, 72.2% initiated pharmacotherapy for depression within this year.
The children ranged in age from 3 years to 6 years, 11 months. Children from the four groups did not differ in gestational age or birth weight (
Table 1). Group 4 children encountered nonsignificantly fewer neonatal complications (data not shown). Among the children exposed to antidepressants, 11.3% received a diagnosis of poor neonatal adaptation signs, which are defined as a series of behaviors observed in the neonate following gestational antidepressant exposure (i.e., jitteriness, tachypnea, poor tone, respiratory distress, weak or absent cry, and/or desaturation on feeding) (
12,
13). All children had similar anthropometric measurements (
Table 1).
The children’s intelligence test results revealed no statistically significant difference in the children’s full-scale, verbal, and performance IQs between group 3 and the venlafaxine and SSRI-exposed groups. Children from group 4 had significantly higher full-scale and verbal IQs than those in groups 1 and 2 and significantly higher performance IQs than group 2 children (
Table 1).
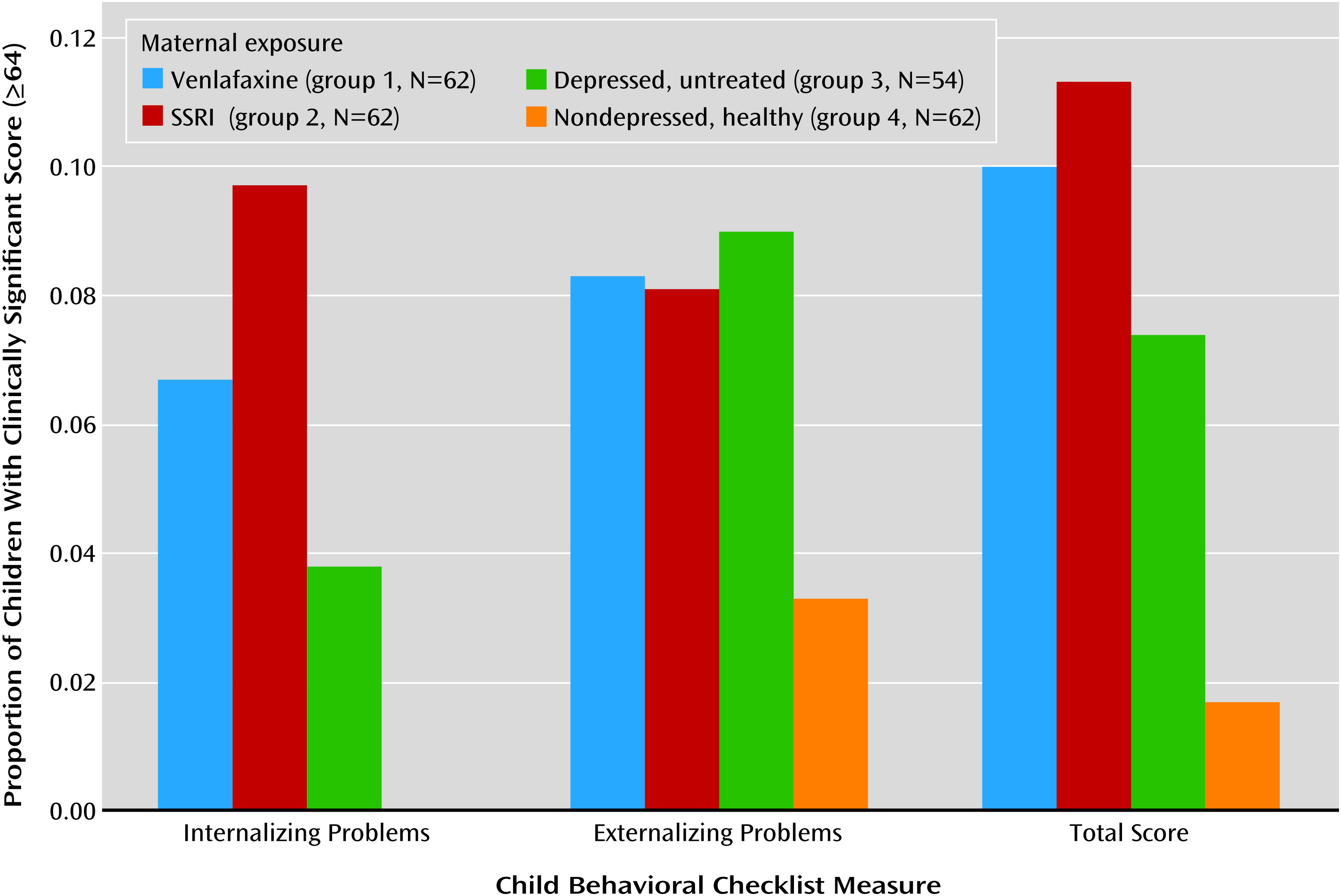
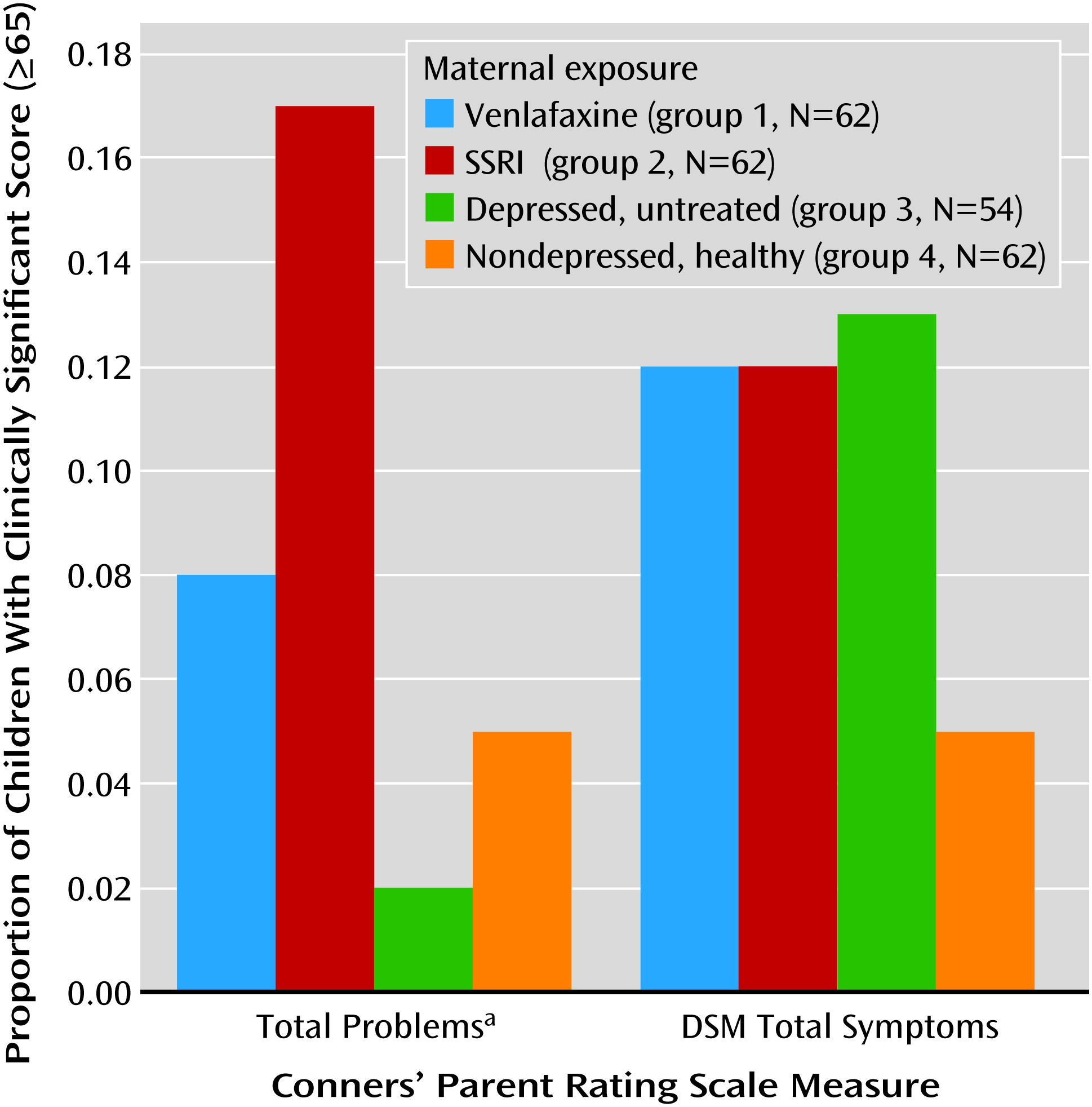
In all groups, girls had statistically significantly higher values on all three IQ measures than boys (data not shown). Children with first-trimester antidepressant exposure had IQs similar to those of children exposed throughout pregnancy (mean full-scale IQ: 103 and 105, respectively; verbal IQ: 106 and 107; performance IQ: 99 and 103). The full-scale, verbal, and performance IQs of the children with poor neonatal adaptation signs were not different from those of the other children exposed to antidepressants. Children in groups 1, 2, and 3 had more clinically significant behavioral problems as assessed with the Child Behavior Checklist internalizing, externalizing, and total problems subscales (total score ≥64) and on the Conners’ Parent Rating Scale total and DSM-IV total symptom indices (total score ≥65) (
Figures 1 and
2). The difference among groups reached statistical significance on the Conners’ Parent Rating Scale score for total problems.
Among the antidepressant-exposed children, clinically significant behavior problems (measured by the Child Behavior Checklist and Conners’ Parent Rating Scale) were twice as common in those with poor neonatal adaptation signs as compared to the other antidepressant-exposed children (14.3%–21.4% versus 6.5%–11.4%).
No significant correlations were found between dose or duration of antidepressant treatment during pregnancy and cognitive and behavioral outcomes (r=0.01–0.12 for dose, r=–0.08–0.14 for duration of treatment). A negative correlation was found between duration of antidepressant treatment and severity of depression in pregnancy (r=–0.23, N=124, p=0.01).
Regression analyses revealed that maternal IQ significantly predicted all three child IQ outcomes and that child sex significantly predicted the child’s full-scale and verbal IQs. Dose and duration of pharmacotherapy for depression during pregnancy, severity of maternal depression during pregnancy and at testing, and child’s age at testing were not significant predictors of cognitive outcomes in these models (
Table 2). After we controlled for other factors, group membership did not predict cognitive outcomes.
The difference between maternal IQ and the child’s full-scale IQ was predicted by maternal IQ alone. Child’s sex, child’s age at testing, dose and duration of antidepressant exposure during pregnancy, and severity of depression during pregnancy and at testing did not predict this outcome (
Table 2).
Child Behavior Checklist scores for internalizing, externalizing, and total problems were all significantly predicted by severity of maternal depression during pregnancy and at the time of testing (
Table 2). The Conners’ Parent Rating Scale total index was predicted by severity of maternal depression during pregnancy and at the time of testing, while the score for DSM total symptoms was predicted by severity of maternal depression during pregnancy alone. Dose and duration of maternal antidepressant treatment during pregnancy, child’s sex, child’s age at testing, and maternal IQ were not predictors of these outcomes (
Table 2).
We tested the interactions between severity of maternal depression during pregnancy and each of the other measures included in the model. None of these interactions produced a better model or was statistically significant.
Discussion
To our knowledge, this is the first study designed to examine, as a primary outcome, the intelligence of children born to mothers taking antidepressants during pregnancy in relation to the intelligence of children of mothers with untreated depression and children of nondepressed mothers.
Children from the venlafaxine and SSRI groups did not differ significantly from children of mothers with untreated depression in any of the cognitive outcome measures (full-scale IQ, verbal IQ, and performance IQ) or behavioral measures (Child Behavior Checklist or Conners’ Parent Rating Scale subscales). There was no difference in full-scale, verbal, or performance IQ between the children in the venlafaxine and SSRI groups. The children of the healthy women had higher IQs than those exposed to antidepressants. Children from all three groups exposed to maternal depression had more clinically problematic behavior and temperament scores than the children of the nondepressed women (
Figures 1 and
2), although the difference among groups reached statistical significance only for the score on the Conners’ Parent Rating Scale total problems subscale.
The differences between maternal IQ and child IQ were similar in the four groups. Dose and duration of antidepressant treatment were not found to be predictors of this outcome in regression analysis, suggesting no treatment effect (
Table 2).
Significant predictors for the child’s IQ were maternal IQ and child’s sex, while a significant predictor for all behavior and temperament outcomes was the severity of maternal depression during pregnancy and/or at the time of testing (
Table 2). None of these results was predicted by dose or duration of antidepressant treatment in pregnancy.
Previous studies found no differences in neurodevelopmental outcomes between antidepressant-exposed children and comparison groups or general population norms. However, these studies were limited in a number of ways: 1) they were not specifically designed to assess intellectual outcomes, 2) they lacked appropriate comparison groups, 3) they had small study groups and were thus underpowered, and 4) they did not account for significant confounders, i.e., maternal depression, drugs of abuse, socioeconomic status, and other environmental and genetic factors (
14–
23).
Using the same prospectively collected database as that used in the current study, we previously found no differences in neurodevelopmental outcomes between children of healthy comparison groups and children exposed to fluoxetine and tricyclic antidepressants (
14,
15). The use of a mixed test battery and participants with a broad age range, including primarily younger children, in the previous study may explain the different results.
We found that even in the exposed group, child IQ levels were considerably higher than the normative mean of the general population, set at 100 by the test. The high IQs in the current study may reflect the secular upward drift in IQ known as the Flynn effect (
24). Furthermore, our study group was derived from a large, urban Canadian city; mothers who contacted Motherisk may have had higher IQs than those who did not, and the Canadian norm for full-scale IQ is five points higher than the usual norm for the Wechsler test we used (
7). We accounted for possible genetic and environmental confounders, including socioeconomic status, in the statistical analysis. Also, this cohort should not be different from the general population in their physiological response to drugs. This may support the generalizability of our results.
The 14 children with poor neonatal adaptation signs did not differ in intelligence from the other exposed children, showing that these transient neonatal signs did not have a long-lasting impact on child cognitive development in this cohort. Nevertheless, one-fifth of these children had clinically significant behavior problems, suggesting that these signs may be a risk factor for future psychopathology. Further study is needed to confirm these findings.
Although the current study showed differences in full-scale, verbal, and performance IQs between the antidepressant-exposed children and the children of the healthy mothers, the differences were accounted for by maternal IQ and child’s sex and not by drug exposure. Furthermore, there were no differences in intelligence outcomes between children exposed during the first trimester of pregnancy and those exposed throughout pregnancy, suggesting that prolonged gestational exposure to antidepressants did not influence cognitive development.
Across all four groups, girls had higher IQs than boys, which is consistent with past findings concerning children between the ages of 4 and 6.5 (
25). Although sex differences in brain development and maturation remain poorly understood, recent neuroimaging and cognitive performance studies provide some possible explanations. Research by Lenroot et al. showed in 387 subjects that while mean total cerebral volume is around 10% larger in males than females, peak volumes in important brain structures occur earlier in females than males (8.5 years versus 10.5 years for gray matter, 10.5 years versus 14.0 years for the caudate nucleus, and 10.5 years versus 14.5 years for total cerebral volume) (
26). From a cognitive performance perspective, studies have shown that females excel at verbal and perceptual tasks, while males perform better on visuospatial exercises (
27).
In the present study, differences between maternal IQ and child IQ were similar in the two sexes across all groups. This suggests that sex differences in brain development, but not treatment, may explain the observed sex differences in IQ.
Regression analysis revealed that maternal IQ was a significant predictor of all children’s IQs. However, despite similar maternal IQs across all four groups, the depression-exposed children had lower IQs than the children of the healthy women, although the differences were not significant in the children of the women with untreated depression. Other factors, such as a less interactive and supportive environment associated with maternal depression, may be influencing their IQ results. Maternal depression may increase the propensity for prenatal psychobiological stress, which is also associated with adverse long-term outcomes in the child (
28). Depression-associated stress and anxiety are thought to disrupt maternal programming for fetal neurodevelopment (
29), leading to later intellectual deficits due to inhibition of neurogenesis and decreased gray matter (
30). The negative correlation found between duration of antidepressant treatment and severity of depression in pregnancy suggests that managing maternal depression may help to minimize these effects.
In this cohort, the women in the three depressed groups had experienced depression for the same number of years, but those with untreated depression had received fewer years of pharmacotherapy. This supports the idea that women who receive pharmacotherapy during pregnancy may suffer from a different, more severe form of depression than those who do not (
31), which may explain our findings.
Eighty percent of the women who discontinued pharmacotherapy during pregnancy experienced an episode of depression in the first year after delivery; this result supports previous findings highlighting the significance of discontinuation of pharmacotherapy (
32). As a large body of research shows that postpartum depression is a significant risk factor for impaired cognitive and emotional functioning (
33) in children, the importance of treating maternal depression in pregnancy is hard to overestimate.
On the Child Behavior Checklist and the Conners’ Parent Rating Scale behavioral problem questionnaires, we found that children from all three groups of depressed mothers exhibited more problematic behaviors than children of the healthy mothers. Severity of maternal depression during pregnancy and/or at the time of testing significantly predicted results on tested subscales, whereas dose and duration of antidepressant treatment during pregnancy did not. This supports earlier studies showing that maternal mood, rather than drug exposure, predicted the child’s internalizing and externalizing behaviors (
22,
23).
Notably, in the present study, the Child Behavior Checklist and Conners’ Parent Rating Scale questionnaires were completed by the mother, whose perception of her child may have been negatively affected by depression and its associated anxiety and stress. The strong correlation between maternal and paternal ratings of a child’s behavior on the Child Behavior Checklist (r=0.5) (
34) will allow future research to corroborate our findings with results gathered from other caregivers or teachers.
Family history of mental illness is also a significant risk factor for subsequent affective problems (
35,
36) and externalizing behaviors in children (
37,
38). Having one depression-affected parent increased the risk of depression in offspring by up to threefold (
39). Goodman and Gotlib (
36) proposed four mechanisms for transmission of maternal depression to the child: a genetic predisposition to mental illness passed on through maternal genetic material, prenatal depression-associated abnormalities in fetal development, exposure to and learning of negative or maladaptive emotions and behavior from living with a depressed mother, and the child’s sociocontextual environment, which may offer low social support and increased parental stress and familial conflict.
Through composite genetic and environmental influences, parental IQ is known to strongly predict children’s IQ (r=0.41–0.45) (
40). In the present study, maternal IQ alone was assessed because of practicality. Additionally, there is evidence that maternal IQ is more strongly correlated with the child’s IQ than is paternal IQ and that maternal and paternal IQs are strongly correlated with each other (
40).
This study’s strengths include the prospectively obtained, accurate information regarding pregnancy exposures and the collection of data at 6–9 months after delivery, which serves to reduce recall bias. Clear inclusion and exclusion criteria, masked psychological assessments, and standardized and validated psychological tests were also used. The presence of an untreated depression group served to help separate the effects of maternal depression from the potentially teratogenic effects of the drugs, and we accounted for many possible environmental and genetic confounders.
Study limitations included maternal self-report of depression in pregnancy and the assessment of child behavior problems through questionnaires, which may be contaminated by maternal mood. Additionally, the children were assessed at only one time point, which could have limited our results, as behavioral patterns and disturbances, as well as IQ, may change over time.
Overall, our study failed to find an effect of antidepressant medication on children’s intellectual or behavioral outcomes. Rather, we documented that untreated depression is associated with a high risk for postpartum depression and that fetal and childhood exposure to maternal depression were significant predictors of child behavior problems and may represent risk for long-term child psychopathology. Further research is warranted to support these findings.