We evaluated two hypotheses about the association between adult levels of nicotine dependence and age at onset of smoking, which we term “noncausal” and “causal.” If the correlation between onset age for smoking and subsequent levels of nicotine dependence is not causal but rather results from social or temperamental factors acting on both variables, the noncausal hypothesis would predict little association between age at onset of smoking and later nicotine dependence within our monozygotic twin pairs. This is because both members of each pair will be quite similar in their social background and temperament. However, if the observed correlation between age at onset of smoking and later nicotine dependence is causal, then we would predict, even within our closely matched monozygotic pairs, a strong association between age at smoking onset and levels of subsequent nicotine dependence.
We also wished to test for the specificity of this effect. If within our monozygotic twin pairs the early-onset smoking member does have an elevated risk for nicotine dependence, would that be specific to nicotine dependence, or might we also see an elevated risk for use and abuse of, and dependence on, alcohol and level of use of a range of illicit substances?
Method
Participants in this study derived from two interrelated studies of Caucasian same-sex twin pairs who participated in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (
16). All subjects were ascertained from the population-based Virginia Twin Registry, which was formed from a systematic review of birth certificates in the Commonwealth of Virginia. Female-female twin pairs born between 1934 and 1974 were eligible if both members responded to a mailed questionnaire in the period 1987–1988. For this study, data were obtained from the third (1992–1995) and fourth (1995–1997) interview waves. Cooperation at the first two waves was in the range of 92%–93% (
16); for the third and fourth waves, we succeeded in interviewing 88% and 85%, respectively, of eligible twins. Data on the male-male pairs came from a sample (birth years 1940–1974) initially ascertained directly from registry records containing all twin births; the cooperation rate was 72%. The first interview was completed largely by telephone between 1993 and 1996. The second wave of interviews was conducted between 1994 and 1998, with a response rate of 83%. All the data for these analyses come from the wave 2 data. After an explanation of the research protocol was given, signed informed consent was obtained for face-to-face interviews and verbal consent for telephone interviews. This project was approved by the Office of Research Subjects Protection at Virginia Commonwealth University. Members of a twin pair were always interviewed by different interviewers.
Zygosity was determined by discriminate function analyses using standard twin questions validated against DNA genotyping in 496 pairs (
17). The twins’ mean age and years of education were 36.3 years (SD=8.2) and 14.3 years (SD=2.2), respectively, at the wave 4 interview of female-female pairs, and 37.0 years (SD=9.1) and 13.6 years (SD=2.6), respectively, at the wave 2 interview of male-male pairs.
Age at onset of regular smoking was assessed by the interview question “How old were you when you began to use tobacco regularly?” Nicotine dependence was assessed by the Fagerström Test for Nicotine Dependence and by an assessment of intensity of craving for the time in participants’ lives when they reported smoking most heavily (
18). The wording of the item assessing craving was as follows: “During this time when you smoked most heavily, if you didn’t smoke for a period of time, how strong would your craving get for another? Would you say very strong, strong, moderate, or hardly any craving?”
Our main statistical analysis was conducted by paired t tests for continuous measures and McNemar chi-square tests for dichotomous variables. We used one-tailed p values, as we had a clear directional hypothesis that the early-onset smoker in each pair would have greater levels of nicotine dependence and other drug use and misuse than his or her later-onset co-twin. For our analyses of maximal illicit substance use, if the twin denied ever trying the substance, they received a score of zero. Effect sizes were estimated using Cohen’s d statistic, for which values of 0.2, 0.5, and 0.9 are considered small, medium, and large effects, respectively (
19).
A priori, we decided to analyze these pairs separately and consider as positive results that broadly replicated across both samples. The female-female sample was considerably smaller and had less statistical power. Therefore, we analyzed the male-male sample first and then considered a result replicated if the effect size (rather than the p value) was similar in the female-female pairs.
Discussion
We sought to evaluate two hypotheses about the nature of the correlation between age at onset of smoking and subsequent nicotine dependence. To do this, we selected, from a large population-based twin sample, monozygotic twin pairs in which both members had a lifetime history of regular smoking and one twin (the early-onset member) reported the onset of regular smoking at least 2 years prior to his or her co-twin (the late-onset member). We identified 175 male-male and 69 female-female pairs who met this definition. On average, the early-onset members reported starting to smoke 4–5 years before the later-onset co-twin.
Our noncausal hypothesis predicted that within these monozygotic twin pairs, we would see no systematic differences in levels of nicotine dependence in the early-onset member compared with the late-onset member. By contrast, the causal hypothesis predicted that the association would still be observed in these pairs despite the fact that the early-onset member and the late-onset member were matched for genotype and social background.
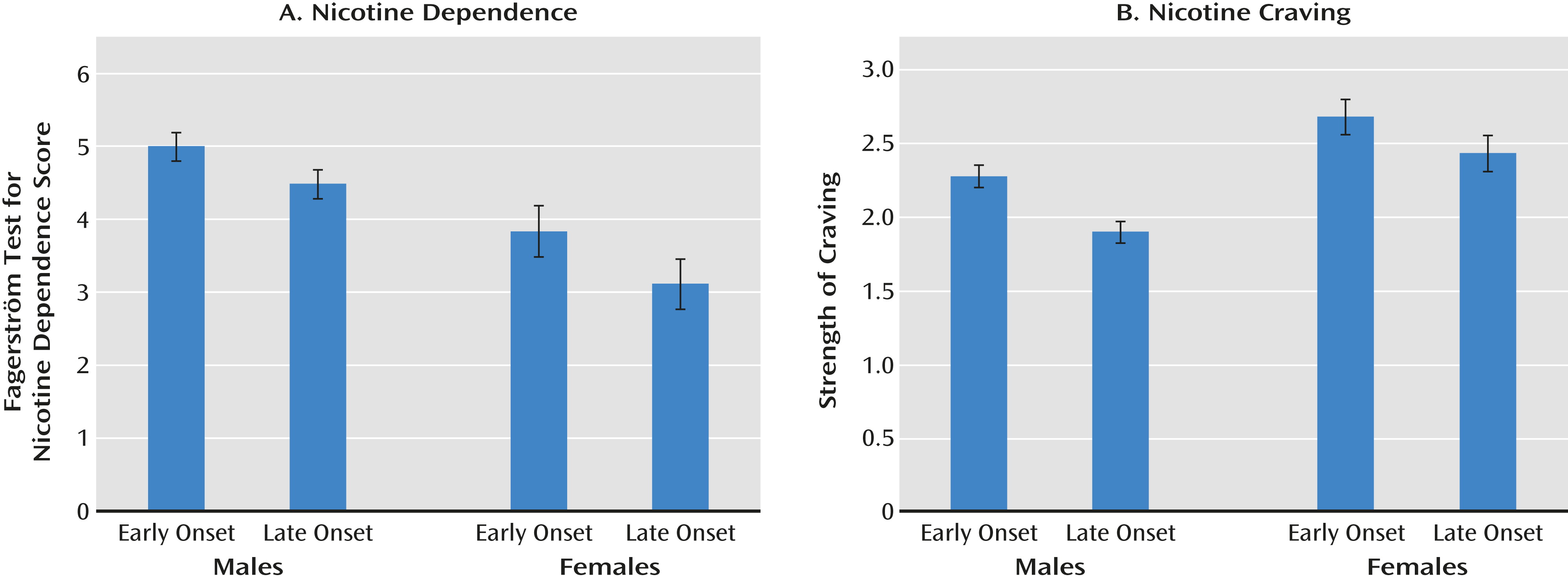
Our results supported the causal hypothesis. Compared with the late-onset members, the early-onset members reported significantly higher levels of nicotine dependence as assessed both by the Fagerström Test for Nicotine Dependence and by levels of craving. The results were consistent across the male-male and female-female twin pairs, supporting the validity of our findings.
We then examined in these pairs whether early-onset smoking predisposed to the heavy use and misuse of other commonly used psychoactive substances. In neither the male nor the female twin pairs was there any consistent evidence that the early-onset member drank more alcohol, had more symptoms of alcohol abuse or dependence, or consumed more illicit substances than the late-onset member.
These results suggest but do not prove that the association between early exposure to nicotine and later symptoms of nicotine dependence is a causal one. The power of the monozygotic co-twin control design is that it controls for any genetic or familial-environmental confounding effects—both those that we could measure and those of which we could not even conceive. Our results rule out the possibility that the association we are seeing between age at first smoking and subsequent levels of nicotine dependence results from the effects of genetically influenced temperamental variables that have an impact both on age at onset of smoking and on risk for nicotine dependence, as has been seen for early-onset drinking and subsequent levels of alcohol consumption (
9,
11). This is critical because in general population samples, we and others have shown that there are shared genetic risk factors between smoking initiation and subsequent levels of nicotine dependence (
9,
21) and smoking persistence (
22). Furthermore, Korhonen et al. (
23) found in a longitudinal study that the effects of hyperactive-impulsive traits (known to be substantially heritable [
24,
25]) on illicit drug use were mediated through early smoking. But such genetic effects cannot be operating within our monozygotic twin pairs. Furthermore, low socioeconomic status influences both age at regular smoking and risk for nicotine dependence, as well as related constructs, such as heavy or persistent smoking (
9,
26,
27). Such effects, however, could not have an influence on the association between age at onset of smoking and nicotine dependence in our pairs because they all grew up together.
Despite its methodological strengths, however, this study is purely observational, and the confidence in causal inference can never approach what is possible with randomized trials. In particular, we cannot rule out the possibility that the risk factor and the outcome (here, early-onset smoking and nicotine dependence) are associated because both resulted from environmental experiences occurring to one member of the twin pair and not the other. We were able, at least partly, to address the plausibility of this explanation and found that the early-onset members were at increased risk only for nicotine dependence and not for the use or misuse of other psychoactive substances. It is difficult to imagine an exogenous environmental experience unique to one twin of a pair that could be so specific in its effect—that would increase risk for nicotine dependence and for no other form of substance use or misuse.
Our data set also contained information about the experiences of childhood physical and sexual abuse, in both the male-male and female-female twin pairs (
28,
29), which are associated with an elevated risk for a range of adverse psychiatric outcomes, including heavy smoking and nicotine dependence (
30–
32). This gave us the opportunity to evaluate empirically the possibility that our findings resulted from these severe environmental exposures. In a fixed-effects (conditional) linear regression implemented in PROC GLM in SAS (SAS Institute, Cary, N.C.), the associations between onset of smoking and nicotine dependence were effectively unchanged in both male-male and female-female pairs when controlling for childhood physical and sexual abuse. Of course these results do not address the possibility that our study results emerged from the impact of other environmental exposures unique to one member of these monozygotic pairs.
The other key potential limitation of this study is its reliance on long-term recall. Could some correlated memory bias cause one twin in each pair to both move back in time their age at onset of smoking and exaggerate their subsequent levels of nicotine dependence? We did assess, over a mean of approximately 50 months, the test-retest reliability both for age at onset of regular smoking (N=962) and for total score on the Fagerström Test for Nicotine Dependence (N=846) in our male-male twin pairs. Both were high (r values, 0.79 and 0.76, respectively). While we cannot rule out the possibility that our findings result from correlated errors of recall, in the light of the highly reliable nature of memory for our key exposure and outcome variables, this seems unlikely.
Our results in humans are consistent with animal studies, which show that exposure to nicotine earlier in life renders experimental subjects at higher risk for nicotine dependence later in life. Exposure of female and male rats and mice to nicotine in early adolescence, but not in adulthood, is associated with greater nicotine self-administration and conditioned reward and preference as manifested in adulthood (
5,
8,
33).
Furthermore, studies in rodents have suggested possible biological mechanisms for the results of the present study. For example, gene expression profiling analyses in rats comparing nicotine exposures during adolescence and adulthood found that many genes, in particular those that influence neuroplasticity, showed persistent changes in brain regions involved in nicotine’s reinforcing and rewarding, such as the ventral tegmental area, only when nicotine was administered during adolescence (
34,
35). In addition, these studies suggest that these effects could be mediated through the regulation of brain α
4β
2 nicotinic receptors. These receptor subtypes play a crucial role in nicotine dependence and are the primary target for varenicline, a smoking cessation drug. Higher levels and functioning of these nicotinic receptor subtypes were observed in the midbrain and striatum of rats and mice exposed to nicotine during adolescence than those exposed only in adulthood (
5,
33). Overall, animal studies show that nicotine exposure during adolescence induces long-lasting biochemical, anatomical, molecular, and behavioral changes that differ substantially from those seen with adult exposure.
If correct, our findings have implications for public policy. It has already been established that nicotine dependence is the primary cause of chronic cigarette consumption, which in turn is responsible for a wide range of health problems and premature mortality. It therefore follows that if early age at first smoking is causally related to subsequent levels of nicotine dependence, then reducing access to tobacco products for adolescents will reduce the total population burden of nicotine dependence and the substantial associated morbidity and mortality. This problem has an added urgency with the increasing rates of smoking worldwide in what has been termed the globalization of the tobacco epidemic, which is driven in part by youth exposure to tobacco-friendly messages in a variety of public media (
36). While our findings suggest that delaying onset of smoking by 2 or more years will produce only a modest reduction in levels of maximal nicotine dependence in adulthood at the individual level (Cohen’s d between ∼0.2 and 0.4, a small to medium effect size), such effects when extrapolated to a population level would result in a substantial total reduction in nicotine dependence and smoking-related disease burden. Our findings reinforce the importance of efforts already under way (
37,
38) to try to ensure that the developing brains of adolescents are protected, in so far as possible, from exposure to the nicotine in tobacco products.