Restricting-type anorexia nervosa is a severe eating disorder associated with malnutrition, underweight, and high mortality. It is distinct from bulimia nervosa, which is characterized by regular binge eating and purging episodes but normal weight. Both disorders usually begin during adolescence, occur most commonly in females, and aggregate in families.
Functional brain imaging has implicated the striatum, insula, anterior cingulate, amygdala, and orbitofrontal cortex in eating disorders (
1). The underlying mechanisms for alterations in these structures are unclear, but gray and white matter may be directly related to altered brain function and behavior (
2).
Findings from research on brain structure in eating disorders have been inconsistent. Early studies suggested reduced total gray and white matter volume, and studies in patients who had recovered from eating disorders found reduced or normal total brain tissue volumes (
3). For the study of regionally specific volume alterations, brain analysis methods have become available that allow automated whole-brain comparison, reducing bias (
3). A systematic review (
3) found eight such studies in adults, and another has been published since (
4). Those studies suggested reduced gray matter volume in anorexia nervosa in the insula, frontal operculum, and occipital, medial temporal, or cingulate cortex, and one recent study found
increased gray matter volume in the dorsolateral prefrontal cortex (
5–
8). After short-term recovery, individuals with anorexia nervosa have shown reduced gray matter in the insula, striatum, and occipital, frontal, and parietal cortex (
8), but brain tissue seems to increase with weight gain (
9) and has been shown to be normal after long-term recovery (
10). The few studies in patients with bulimia nervosa suggested normal or increased localized gray matter volume in the orbitofrontal cortex and striatum (
4,
6). These variable results may reflect the heterogeneity of approaches. Only some studies corrected for age or overall brain volumes; some studies distinguished the restricting type from the binge eating and purging type of anorexia nervosa while others did not; and the effects of comorbid diagnoses or medication were often not directly taken into account.
Individuals ill with and recovered from anorexia nervosa show increased eating concerns, as do individuals with bulimia nervosa between binge episodes (
11). Notably, affective value attributed both to food stimuli and to food avoidance (
12) has been associated with medial orbitofrontal cortex function (
13). Furthermore, orbitofrontal cortex function has been directly associated with taste pleasantness (
14), which could have implications for sensory-specific satiety in eating disorders and being quickly overstimulated by a food type. Orbitofrontal function has been repeatedly associated with brain pathology in anorexia nervosa and bulimia nervosa, including in studies using food valence ratings (
13,
15,
16), and could be a key area of brain pathology in eating disorders. On the other hand, brain reward function indicated
opposite response in the striatum and insula in anorexia nervosa and bulimia nervosa (
17,
18), and those regions might therefore distinguish eating disorder groups.
Methodological problems in eating disorders brain research can include inaccurate brain alignment or separation of gray and white matter in imaging as a result of brain shapes that do not conform with standard brain templates. Whole-brain structural studies in eating disorders have most commonly used voxel-based morphometry (VBM) and statistical parametric mapping software (SPM;
http://www.fil.ion.ucl.ac.uk/spm/), which analyze gray and white matter probability across the entire brain. Recently the VBM8 toolbox was developed to address shortcomings of the previous versions. It uses a new image registration algorithm and a template based on the individual study population without relying on standard template assumptions, and it shows improved separation of gray and white matter compared with previous VBM versions (
19,
20).
In this study, we wanted to compare individuals ill with and recovered from anorexia nervosa to identify potential trait alterations in the disorder, but also to compare individuals with anorexia nervosa and bulimia nervosa in order to identify brain alterations across eating disorders. We expected the orbitofrontal cortex to show common abnormalities across all eating disorder groups, possibly related to hedonic taste perception (
13,
16), and we expected insula and striatum structures to differentiate the eating disorder types (
3,
17).
Method
Participants
Nineteen women with restricting-type anorexia, 24 recovered from restricting-type anorexia nervosa, and 20 with bulimia nervosa, as well as 24 age-matched healthy comparison women, participated in the study. Participants in the eating disorder groups were recruited from the Children’s Hospital Colorado and the Eating Disorders Center of Denver. The study was approved by the Colorado Multiple Institutional Review Board. Participants in the eating disorder groups were within 1–2 weeks of closely supervised inpatient or partial hospitalization treatment and followed the program meal plan to avoid acute effects of starvation and dehydration (see the
data supplement that accompanies the online edition of this article). Women in the healthy comparison group and those in the recovered anorexia nervosa group were recruited through local advertisements. The Structured Clinical Interview for DSM-IV (
21) was administered by a doctoral-level interviewer. Women in the recovered group had a history of restricting-type anorexia nervosa but had normal weight for height, menstrual cycle, exercise, and food intake for at least 1 year. All participants were right-handed, with no history of head trauma, neurological disease, major medical illness, psychosis, or substance use disorders. Thirteen women in the healthy comparison group, one in the anorexia nervosa group, five in the bulimia nervosa group, and seven in the recovered group took birth control pills. All participants provided written informed consent after receiving a complete description of the study.
Behavioral Measures
Participants completed the Eating Disorder Inventory–3, the Temperament and Character Inventory, the Spielberger State and Trait Anxiety Inventory, the Beck Depression Inventory–II, and the Revised Sensitivity to Reward and Punishment Questionnaire. In addition, participants completed a taste perception test prior to brain imaging in which they rated a 1-molar sucrose solution for sweetness and pleasantness on 9-point Likert scales (see the online
data supplement).
MRI Acquisition and Image Analysis
Structural brain images were acquired on a GE Signa 3-T scanner, with axial three-dimensional T1-weighted magnetization-prepared rapid acquisition gradient echo (spoiled gradient recall [SPGR], field of view=22 cm, flip angle=10°, slice thickness=1.2 mm, scan matrix=256×256, TR=10 ms, TE=3 ms, voxel size=1.2 mm3).
Images were manually aligned on the anterior-posterior commissure line. Preprocessing of T
1-weighted images was performed using the SPM VBM8 toolbox (
http://dbm.neuro.uni-jena.de/vbm/download/) in MATLAB R2009b, 7.9.0 (MathWorks, Natick, Mass.).
VBM8 brain segmentation (see the online
data supplement) does not require a priori tissue probabilities information. After segmentation of T
1/SPGR images into three pure tissue classes (gray matter, white matter, and CSF), two additional mixed tissue classes (gray matter-white matter and gray matter-CSF) are estimated using partial volume effects. The result is an estimation of fraction of pure tissue type present in every voxel. Images were smoothed to an 8-mm full-width at half-maximum Gaussian kernel. Nonlinear modulated data were used in the analyses. Images were normalized to Montreal Neurological Institute (MNI) space using high-dimensional diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL).
Total intracranial volume (global tissue volume) was obtained by adding up gray and white matter and CSF volumes from the tissue class images in native space using the VBM8 toolbox.
To confirm VBM results for orbitofrontal gyrus rectus volume, the left gyrus rectus gray matter was traced using MRIcron (by M.E.S., blind to group) (
http://www.mccauslandcenter.sc.edu/mricro/mricron/) from the most inferior orbitofrontal brain slice to the level of the inferior rostral sulcus as the superior border to include the functionally connected agranular and dysgranular layers, and between the olfactory sulcus as the lateral boundary and the medial longitudinal fissure as the medial boundary (
22).
Statistical Analysis
A general linear model whole-brain analysis was used (SPM8), comprising a factorial design with group as a factor with four levels (healthy comparison group, anorexia nervosa group, recovered anorexia nervosa group, and bulimia nervosa group) and age and total intracranial volume as covariates, as well as use of antipsychotics or selective serotonin reuptake inhibitors and comorbid depression or anxiety (each coded 0 or 1 for presence or absence, respectively). Initially, a voxel-wise F test was performed with a significance threshold of 0.001 uncorrected and an extent threshold of 50 voxels to include functionally relevant brain structures, such as the insula taste area and the orbitofrontal cortex. We used anatomical regions defined by the SPM8 Automated Anatomical Labeling atlas (orbitofrontal cortex, insula, caudate, putamen) for small-volume correction (family-wise error corrected p<0.05). Gray matter regional volumes that reached significance within the anatomical region were extracted using the MarsBaR region-of-interest toolbox (
http://marsbar.sourceforge.net/) for post hoc analysis. Similarly, significant white matter regional volumes from the group whole-brain analysis were also extracted. Demographic and extracted regional brain volumes were analyzed using SPSS (IBM-SPSS, Chicago) and analysis of variance (ANOVA). Post hoc group comparisons were analyzed with Dunnett’s T3, and ANOVA with covariates (ANCOVA) used Bonferroni correction for post hoc comparison, verified using bootstrap procedures. Regression analyses assessed relationships between behavioral measures and brain volumes.
Results
The participants’ demographic and behavioral data are summarized in
Table 1. There were no significant differences in mean age between the eating disorder groups and the healthy comparison group, but participants in the recovered anorexia nervosa group were older on average than those in the anorexia nervosa group.
Body mass index (BMI) was lower in the anorexia nervosa group. Measures for eating pathology, mood, and anxiety as well as reward sensitivity were typically elevated in the eating disorder groups. Ratings of sucrose pleasantness were similar across groups, but sweetness rating was greater in the anorexia nervosa group compared with the recovered anorexia nervosa group.
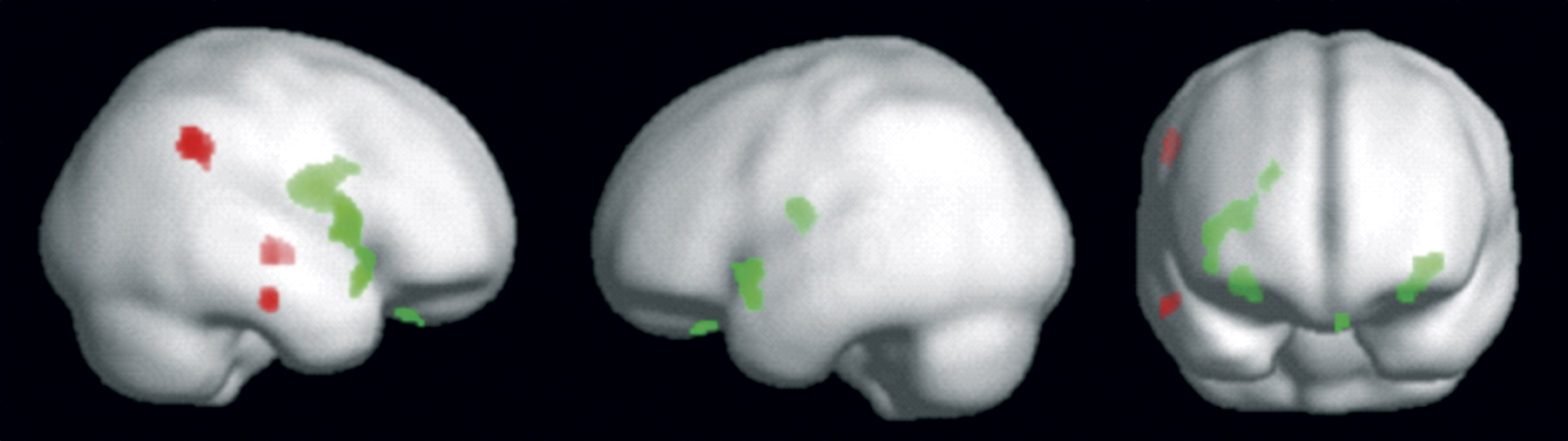
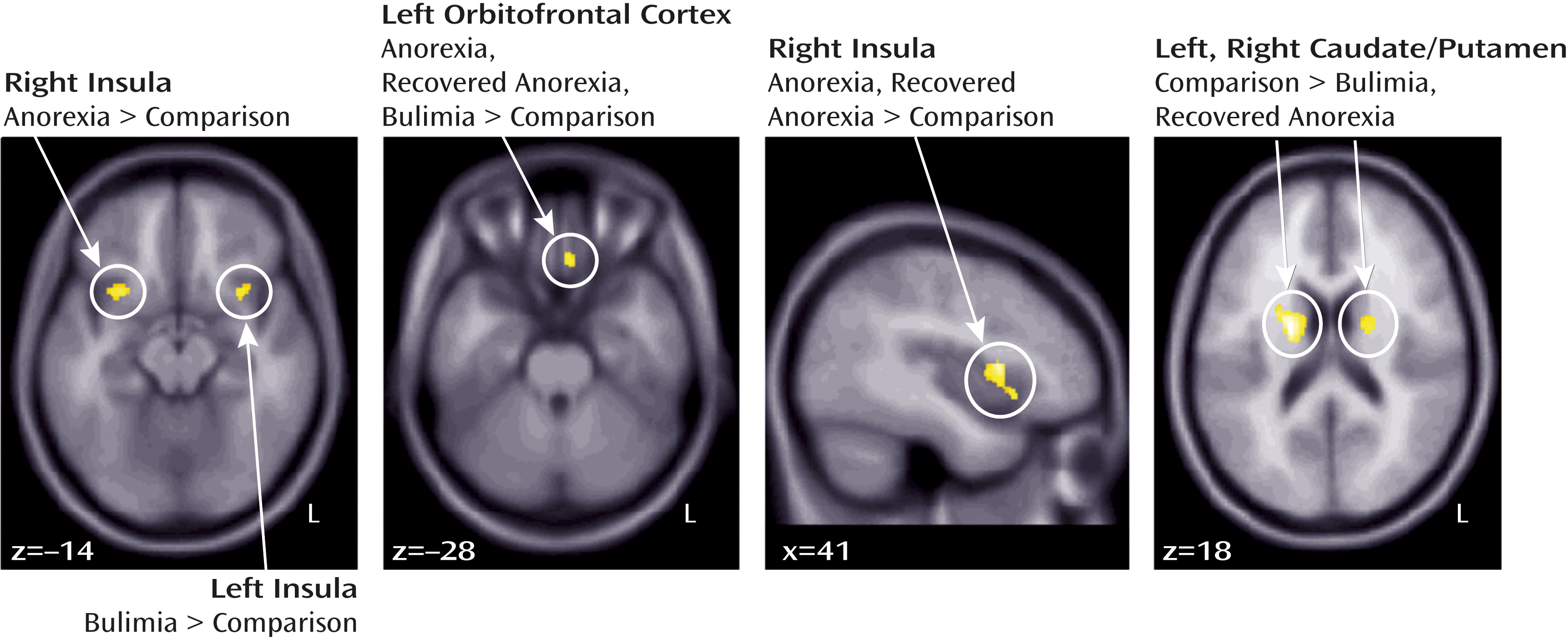
Total brain volume was similar across groups (
Table 2). Gray matter results (
Figures 1 and
2 and
Table 2) indicated increased gyrus rectus volume in all eating disorder groups, reduced caudate and putamen volume in the bulimia nervosa and recovered anorexia nervosa groups, and increased insula volume in the eating disorder groups relative to the healthy comparison group.
An ANCOVA of manually traced gyrus rectus volume (with total intracranial volume as a covariate) confirmed increased volume in the eating disorder groups relative to the healthy comparison group (
Table 2).
White matter results (
Figure 1,
Table 2) showed reduced inferior temporal white matter in the anorexia nervosa and recovered anorexia nervosa groups relative to the healthy comparison group, reduced inferior parietal volume in the bulimia nervosa and recovered anorexia nervosa groups, and reduced medial temporal lobe white matter in the bulimia nervosa group relative to the healthy comparison group.
Results of the regression analyses indicated that age was negatively correlated with gray matter volumes in the healthy comparison group for the right insula (x=30, y=14, y=−12; r=−0.437, p<0.033) and the left gyrus rectus (r=−0.411, p<0.048), in the anorexia nervosa group for the left gyrus rectus (r=−0.830, p<0.001) and the right putamen (r=−0.473, p<0.041), and in the recovered anorexia nervosa group for the left gyrus rectus (r=−0.453, p<0.026).
BMI was negatively correlated with volume in the bulimia nervosa group for the right caudate (r=−0.473, p<0.035) and in the recovered anorexia nervosa group for the left insula gray matter (r=−0.510, p<0.011).
State (r=−0.441, p<0.031) and trait (r=−0.419, p<0.042) anxiety were negatively correlated with left anterior ventral insula gray matter volume in the healthy comparison group but not in the eating disorder groups.
Sensitivity to reward was positively correlated with right putamen gray matter in all eating disorder groups (MNI coordinates, x=20, y=0, z=12, and x=23, y=0, z=15; anorexia nervosa: r=0.620, p<0.005, and r=0.554, p<0.014; bulimia nervosa: r=0.543, p<0.013, and r=0.443, p<0.050; recovered anorexia nervosa: r=0.420, p<0.041, and r=0.397, p<0.055).
Other variables, including duration of illness or recovery or binge/purge episodes, did not predict brain volume measures.
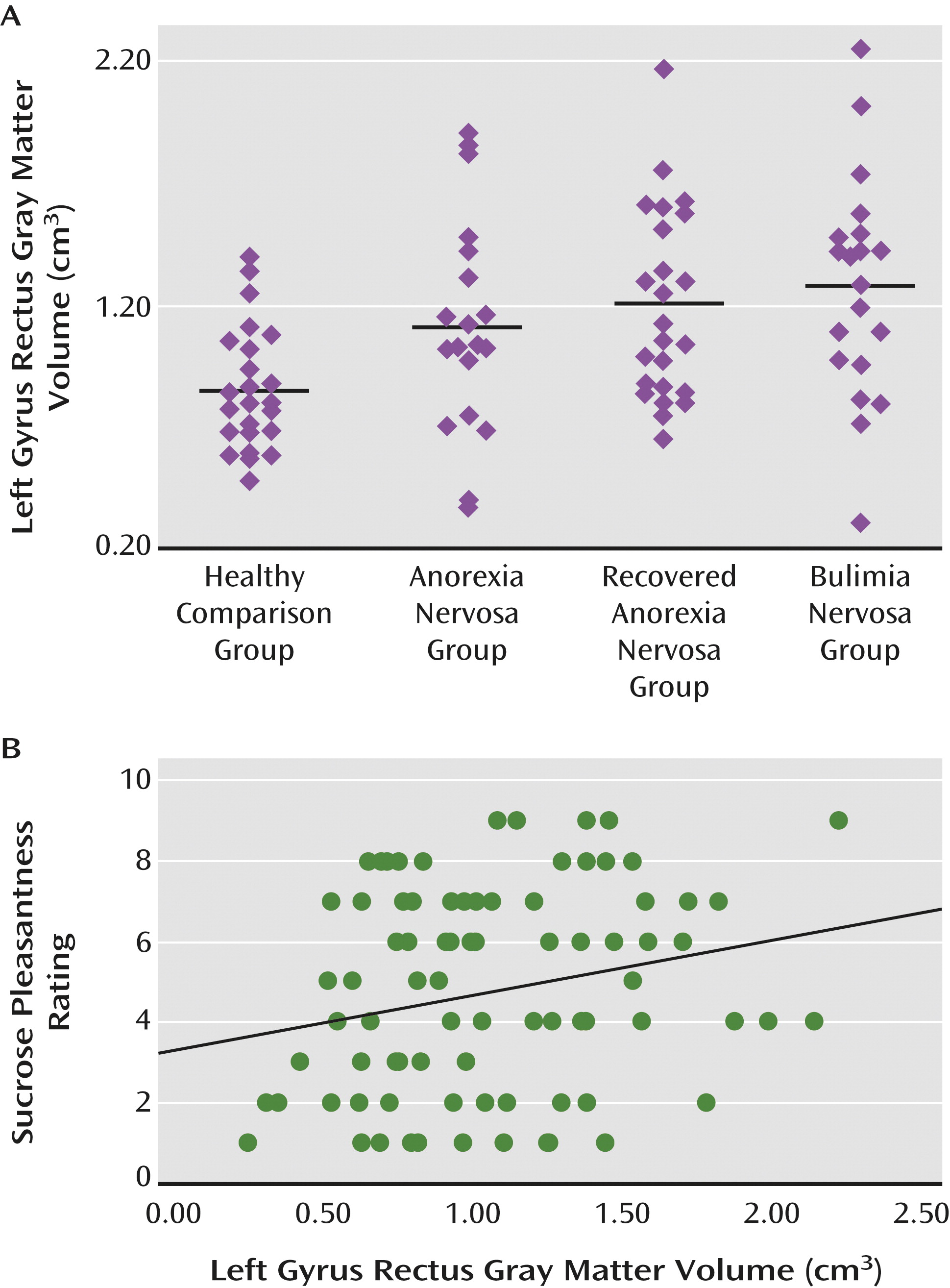
Gyrus rectus volume was positively correlated with sucrose pleasantness rating in the healthy comparison group (r=0.419, F=4.970, p<0.03) and in the eating disorder groups combined (r=0.268, F=4.50, p<0.038), as well as in all study subjects together (Figure 3), but not in the eating disorder groups separately.
There were no significant correlations between white matter volume and BMI or behavioral measures.
Discussion
The results of this large, well-controlled study implicate both overlapping and distinct brain morphology in two phases of anorexia nervosa as well as bulimia nervosa. Findings that show a link between brain structure and both sensitivity to reward and taste pleasantness support the notion that marked neurological underpinnings are associated with phenotypes exhibited across eating disorders. Specifically, VBM results indicate that anorexia nervosa, recovered anorexia nervosa, and bulimia nervosa are associated with increased left orbitofrontal gyrus rectus gray matter volume. Anorexia nervosa is associated with increased anterior ventral insula gray matter volume on the right, and bulimia nervosa on the left; anorexia nervosa and recovered anorexia nervosa are associated with increased gray matter in the right anterior middle insula; and bulimia nervosa and recovered anorexia nervosa, but not anorexia nervosa, are associated with decreased dorsal caudate and putamen gray matter volumes. Notably, in all three eating disorder groups, putamen gray matter was positively related to sensitivity to reward.
White matter was reduced in bulimia nervosa in the medial temporal lobe, in anorexia nervosa and recovered anorexia nervosa in the inferior temporal lobe, and in recovered anorexia nervosa and bulimia nervosa in the parietal lobe.
The results of this adult study are in line with recent reports of normal total gray and white matter volumes (
4,
5) and finds a distinct pattern of increased and decreased regional cortical and subcortical brain volumes in anorexia nervosa, recovered anorexia nervosa, and bulimia nervosa. However, we did not find alterations of the cingulate or temporal cortex as did previous studies (
3).
Several factors distinguish our study from past investigations. First, we used more accurate analysis software (
19,
20), and to our knowledge no study in anorexia nervosa and only one study in bulimia nervosa (
4) has used this method. Improved separation of gray and white matter and reduced white matter volume underlying gray matter may have contributed to findings of reduced gray matter in past studies, which may be supported by our finding of reduced white matter in all three eating disorder groups. Second, we report only gray matter areas that survived stringent anatomical region-based small-volume correction as significant. Third, patients with anorexia nervosa and bulimia nervosa were in a strict inpatient or partial hospital program where they had normal food and fluid intake for 7–10 days before brain imaging and were prevented from binge or purge behavior. Fluid changes significantly affect gray matter changes (
23), and our study protocol helped reduce acute effects of nutritional depletion. Fourth, in a highly conservative approach, we used age, depression and anxiety, medication use, and total intracranial volume as covariates, and to our knowledge this is the first brain imaging study of eating disorders to include all those covariates. Lastly, this is the largest study of restricting-type anorexia nervosa and recovered anorexia nervosa as well as bulimia nervosa on gray and white matter brain structure to date. In summary, our study procedures were stringent, and we believe they contributed to improved results.
All three eating disorder groups had increased left gyrus rectus volume, suggesting that this is potentially a trait marker for anorexia nervosa, and possibly also for bulimia nervosa, although this will need to be studied in bulimia nervosa after recovery as well. These findings support previous research implicating the orbitofrontal cortex across eating disorder groups (
13,
16). The gyrus rectus is the medial part of the orbitofrontal cortex (
22). It is further defined by a caudal agranular and dysgranular layer (area 14) that transitions antero-superiorly into the granular layer (area 11) (
24). The agranular and dysgranular layers have fiber connections to the hippocampus, amygdala, cingulate, and insular cortex (
25), areas important for taste as well as reward, motivation, and emotion processing. In line with those functional aspects of the orbitofrontal cortex may be the finding of gyrus rectus volume predicting the rated pleasantness of the sucrose solution. Greater gyrus rectus volume predicted a stronger pleasantness experience, which is consistent with previous research (
26). The orbitofrontal cortex is important in food intake control (
26). It is possible that the larger gyrus rectus in eating disorders is associated with stronger sensory experience of food stimuli, which could be experienced as overwhelming—as supported by increased reward and punishment sensitivity (
17)—which could trigger cognitively driven food avoidance. Notably, the medial orbitofrontal cortex has been associated with food avoidance (
12), and this region therefore may be a key structure in eating disorder pathology. Eating disorder phenotype differences with restriction in anorexia nervosa and episodic binge eating in bulimia nervosa, by contrast, may be driven by differences in the insula and basal ganglia between the two disorders (
17,
18).
The cause for increased gyrus rectus volume is unclear. One potential explanation is that the trajectory of orbitofrontal gray matter development in eating disorders may be delayed, reaching peak volume later than in healthy comparison subjects and thus resulting in greater cortical thickness and volume (
27). Another possibility could be effects of repeated food restriction in the eating disorder groups, but this will need to be tested further.
Individuals ill with and recovered from anorexia nervosa showed increased volumes in the right anterior ventral/middle insula, which connects to ventral striatal and orbitofrontal reward pathways (
26). Previous research has implicated insula function in eating disorders (
1), and altered insula structure could underlie altered function. The anterior ventral insula is connected to the amygdala (
28) and has been associated with fear response (
29). It also aids in connecting complex perceptual inputs to generate internal emotional states (
30). Thus, altered insula volume could contribute to dysfunction in the regulation of anxiety by the insula, contributing to high trait anxiety in anorexia nervosa. Functional imaging taste reward studies have suggested excessive insula activation in anorexia nervosa (
17), and increased size could mediate excessive, overwhelming taste stimulus transmission and subsequent input into reward-processing brain regions.
The right anterior insula has also been associated with self-recognition—the “abstract representation of oneself” (31)—and interoceptive awareness (32). The fixed perception of being fat while severely underweight in anorexia nervosa (33) could thus be related to right-sided increased anterior ventral insula volume and dysfunction. Left anterior ventral insula activation is related to gastric distention (34) and self-reported fullness (35). Thus, altered anterior insula size could interfere with normal interoception in bulimia nervosa, which may contribute to a reduced ability to sense fullness or satiation and then trigger the urge to purge after excessive food intake and guilt experienced over eating. Dorsal caudate and putamen volumes were reduced in the recovered anorexia nervosa and bulimia nervosa groups, but not the anorexia nervosa group. Nevertheless, in all three eating disorder groups, right putamen gray matter volume correlated significantly positively with sensitivity to reward. The dorsal striatum has been widely associated with supporting rewarding behaviors based on previous experience (
36), and reduced brain volume in that region might alter reward-motivated behaviors. Activation of the dorsal striatum responds to reward and punishment (
37) and contributes to reward-based decision making (
38). It is rich in dopamine D
1 and D
2 receptors, which code reward response, but those receptors have opposing effects (
39). Thus, dopamine receptor expression may be affected by altered dorsal striatal volume and be related to altered sensitivity to reward in eating disorders.
Various right-sided white matter regions showed reduced volume in the eating disorder groups. The functionality of such alterations is unclear, but the fact that the recovered anorexia nervosa group showed reduced volumes in the right temporal and parietal lobe suggests either long-lasting or premorbid volume reductions in anorexia nervosa. The right-sided reduced inferior parietal lobe/temporo-parietal junction area in the recovered anorexia nervosa and bulimia nervosa groups has been associated with fiber paths connecting with the insula, especially in women (
40), further indicating an involvement of insula-related brain circuitry in eating disorders.
Limitations
Although this is the largest structural imaging study contrasting anorexia nervosa, recovered anorexia nervosa, and bulimia nervosa to date, replication is needed. Some of the results are in contrast to previous studies that found reduced brain volumes, although in our study we found both increased and decreased gray matter volumes as well as decreased white matter volumes. The brain analysis method we used shows improved accuracy (
19,
20), and we do not believe our analysis was affected by any methodological systematic error. Notably, this approach does not depend on standard template assumptions but normalizes the images to a template created from the specific study population, thus improving tissue segmentation accuracy. The manual tracing of gyrus rectus volume also supports the whole-brain VBM results. Furthermore, the results in the anorexia nervosa and normal-weight recovered anorexia nervosa groups point toward consistent brain alterations.
The reason for increased gray and reduced white matter volume is unclear. One possibility is the alteration of brain maturation in eating disorders. During development, gray matter in adolescence decreases (indicative of synaptic pruning), beginning in puberty in sensorimotor areas and then spreading during late adolescence into higher-order cortical regions while white matter increases, indicative of thicker myelin sheaths, increased axonal diameter, and improved organization of white matter tracts, improving signal transduction (
27). A delay or incomplete maturation of brain structures in eating disorders could be responsible for the results in this study and would fit the developmental perspective. This points to an interesting future research direction and suggests the need for longitudinal imaging studies in these groups. However, while we made every effort to reduce effects of acute malnutrition, past or more recent effects of underfeeding may also have contributed to the differences across groups. Age, comorbidity, and use of medication are all potential confounders in brain imaging studies. We accounted for those factors by using them as covariates in the imaging analysis, and it is possible that inclusion of the covariates contributed to the fact that we did not find, for instance, alterations in the cingulate or temporal cortex. Sucrose pleasantness ratings were similar across groups, and sweetness perception was similar between the eating disorder groups and the healthy comparison group. However, the anorexia nervosa group rated sweetness higher compared with the recovered anorexia nervosa group. The meaning of this difference between anorexia nervosa groups is uncertain and needs further exploration. Regression analysis between gray matter volumes and demographic and behavioral data suggested various significant relationships. However, most of those results would not have survived correction for multiple comparisons and should be viewed as preliminary until further replication.
In summary, anorexia nervosa, recovered anorexia nervosa, and bulimia nervosa are associated with increased gyrus rectus gray matter volume, which could be a trait-related alteration. The strong correlations between gyrus rectus volume and rating of taste pleasantness may suggest overstimulation in eating disorders to sensory input, possibly contributing to food avoidance. Increased right anterior ventral insula volume in anorexia nervosa and recovered anorexia nervosa and increased left anterior ventral insula volume in bulimia nervosa distinguished the two disorders. Furthermore, the dorsal putamen appears to be important in modulating reward sensitivity in eating disorders. Studies that integrate brain structure and function will be needed to disentangle how brain volume affects behavior in eating disorders.
Acknowledgments
The authors thank Dr. Joel Yager for his very thoughtful feedback and discussion.