Neuropsychological impairment is a core feature of schizophrenia (
1), and understanding the nature and course of neuropsychological functioning in schizophrenia may have important pathophysiological implications. It is widely believed that individuals diagnosed with schizophrenia experience neuropsychological decline from the premorbid to the postonset period, but relatively few studies have examined change in neuropsychological functioning from before to after the onset of schizophrenia. In this study, we provide a rigorous test of neuropsychological changes in schizophrenia using a battery of tests administered during childhood (ages 7, 9, 11, and 13) and adulthood (age 38) as part of an ongoing population-representative longitudinal study.
There is clear evidence of mild neuropsychological deficits among children who later develop schizophrenia (
2). Neuropsychological deficits are even more pronounced among adults diagnosed with schizophrenia. For example, meta-analyses show an average premorbid 8-point IQ deficit (SD=0.50) among individuals who later develop schizophrenia (
3) but a 14- to 21-point IQ deficit (SD=0.90–1.40) among first-episode and chronic schizophrenia patients (
1,
4,
5). These findings suggest that individuals with schizophrenia experience a relative decline in neuropsychological functioning over time from before to after illness onset, with stabilization in neuropsychological functioning thereafter (
6,
7), or at least until older adulthood (
8–
10).
In line with cross-sectional evidence, the few longitudinal studies that have addressed neuropsychological changes in schizophrenia from before to after illness onset have consistently shown evidence of neuropsychological decline (
Table 1). However, these studies suffer from various limitations. First, the majority of the studies are based on clinical samples, which may not be representative of the full population of individuals with schizophrenia (
22). Second, the age at baseline assessment varies considerably, with many studies assessing neuropsychological functioning for the first time in adolescence or adulthood, when prodromal symptoms (and altered neuropsychological functioning) tend to be present (
23–
25). Thus, these studies may underestimate the magnitude of the decline in functioning. Third, only five of the studies included a comparison group, which is needed to provide a rigorous test of change in functioning. Fourth, many of these studies employed different neuropsychological tests across time, making it difficult to ascertain true change in functioning. Fifth, these studies focused exclusively on IQ (or IQ proxies). Since different neural systems underlie performance on different neuropsychological tests, other important mental functions, such as memory and executive function, should be examined as well. Sixth, none of the studies examined whether, in addition to poor IQ test performance, individuals with schizophrenia experience cognitive problems in their daily life.
In a previous report of our population-representative cohort followed prospectively from birth, we showed that children who later developed schizophrenia had IQ deficits, and we mapped changes in the specific mental functions that constitute the IQ across four measurement occasions from ages 7 to 13 years (
26). Now that this cohort has been followed to age 38 and undergone additional neuropsychological testing, we examined change in IQ, as well as more specific neuropsychological functions, from before (ages 7–13) to after (age 38) the onset of schizophrenia using the same measures across time. In the present study, we tested four hypotheses. First, we tested the “IQ decline” hypothesis to determine whether individuals with schizophrenia experience a decline in IQ from before to after illness onset. We compared the group of individuals with schizophrenia to a psychiatrically healthy group to allow for an accurate interpretation of test-retest performance. Second, we tested the “generalized decline” hypothesis to determine whether decline is apparent broadly across different neuropsychological domains: verbal IQ, performance IQ, learning and memory, processing speed, executive function, and motor function. Third, we tested the “specificity” hypothesis to address whether neuropsychological decline is specific to schizophrenia. We compared neuropsychological decline in schizophrenia to decline in three other groups: a persistent depression group, a mild cognitive impairment group, and a group at risk for schizophrenia. We evaluated neuropsychological decline in individuals with persistent depression to test whether decline is common to other psychiatric disorders. Depression is characterized by neuropsychological impairment (
27,
28), but it is not clear whether there is neuropsychological decline from before to after illness onset. We evaluated neuropsychological decline in children with mild cognitive impairment because they, like children with schizophrenia, exhibit cognitive difficulties early in life. However, unlike in schizophrenia, these children do not develop a psychotic condition. We also evaluated neuropsychological decline in “at-risk” individuals who did not develop schizophrenia but who matched those who did on key childhood risk factors (low IQ, family history of psychotic illness, low socioeconomic status). Fourth, we queried third-party informants to test the “everyday cognition” hypothesis that individuals with schizophrenia experience cognitive problems in daily life.
Method
Participants
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behavior of a complete birth cohort of consecutive births between April 1, 1972, and March 31, 1973, in Dunedin, New Zealand. The cohort of 1,037 children (91% of eligible births; 52% boys) was constituted at age 3 years. Cohort families represent the full range of socioeconomic status in the general population of New Zealand’s South Island and are primarily of white European ancestry. Follow-up assessments were conducted at ages 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 95% of the 1,007 living study members underwent assessment in the period 2010–2012.
The study protocol was approved by the institutional ethical review boards of the participating universities, and study members gave informed consent before participating.
Schizophrenia
Schizophrenia was assessed at ages 21, 26, 32, and 38. We previously described the schizophrenia cases up to age 32 (
26,
29,
30), and here we update this information with data from age 38. DSM criteria for schizophrenia were assessed at each age with the Diagnostic Interview Schedule (DIS) (
31,
32). We took several steps to enhance the validity of our research diagnosis. First, we required the presence of hallucinations (not substance use-related) in addition to at least two other positive symptoms. This requirement is stricter than that of DSM-IV, which does not require hallucinations, although requiring them has been shown to reduce overdiagnosis (
33). Second, because self-reports can be compromised by poor insight in schizophrenia, we required objective evidence of impairment resulting from psychosis, as reported by informants and as recorded in the study’s life-history calendars, which document continuous histories of employment and relationships. Third, in our research, the DIS is administered by experienced clinicians, not lay interviewers. These clinicians record detailed case notes. Our staff also rate observable symptoms manifested in affect, grooming, and speech during the full day that participants spend at our research unit. Fourth, participants bring their medications, which are then classified by a pharmacist. Fifth, informants report study members’ positive and negative psychotic symptoms via postal questionnaires. Finally, study members’ parents were interviewed about their adult child’s psychotic symptoms and treatment as part of the Dunedin Family Health History Study (2003–2005). These data, accumulated in the Dunedin study at ages 21, 26, 32, and 38, were compiled into dossiers reviewed by four clinicians to achieve best-estimate diagnoses with 100% consensus. By age 38, 2% of the cohort (N=20) met criteria for schizophrenia and had, according to the multisource information collected in the dossiers, been hospitalized for schizophrenia (totaling 1,396 days of psychiatric hospitalization, according to official New Zealand administrative record searches) or received prescriptions for antipsychotic medications. An additional 1.7% (N=17) met all criteria for schizophrenia, had hallucinations, and suffered significant life impairment but had not, to our knowledge, been treated specifically for psychotic illness. Together, these two groups constituted a total of 37 cases of diagnosed schizophrenia in the cohort. Of these 37 individuals, four died before the age-38 neuropsychological assessment and two declined to participate, leaving an effective group size of 31 for this study.
Of the 31 individuals in the schizophrenia group, the majority (N=17; 55%) had received treatment specifically for psychotic illness. Of those who, to our knowledge, had not received treatment specifically for psychotic illness, nearly all reported receiving treatment for another mental health problem (
Table 2). The two groups appeared similar on a variety of correlates, including adult IQ, personality functioning, substance dependence, and even receipt of government benefits, suggesting that the groups are comparably impaired. The notable exception was that those who had not received treatment for psychotic illness were from families with lower socioeconomic status.
The cohort’s 3.7% prevalence rate of schizophrenia is high and should be understood in the context of three methodological aspects of our study. First, our birth cohort, with a 95% participation rate, allows us to count psychotic individuals overlooked by previous surveys because individuals with psychotic disorders often decline to participate in surveys or die prematurely (
34), and surveys often exclude homeless or institutionalized individuals with psychosis. Second, our cohort members are all from one city in the South Island of New Zealand. It is possible, given geographical variation in rates of schizophrenia (
35–
37), that the prevalence is somewhat elevated there. No data exist to compare prevalence rates of schizophrenia in New Zealand to rates in other countries, but the high prevalence of suicide in New Zealand could be consistent with an elevated prevalence of severe mental health conditions (
38). Third, our research diagnoses did not make fine-grained distinctions among psychotic disorders (e.g., schizophrenia versus schizoaffective disorder). Thus, the cohort members diagnosed with schizophrenia here might not be considered by all clinicians to have schizophrenia. We note, however, that over half of those we diagnosed were confirmed by receipt of treatment. Moreover, etiological mechanisms appear to be similar across the continuum of psychosis (
39).
Persistent Depression
Depression was assessed by DSM criteria at ages 18, 21, 26, 32, and 38 using the DIS. Cohort members who were diagnosed with depression on two or more occasions between ages 18 and 38 were classified into the persistent depression group. We focused on persistent depression in an effort to make this group more comparable to the schizophrenia group in terms of chronicity and severity of illness. Neuropsychological data were incomplete for six of the 191 cohort members in the persistent depression group, leaving an effective group size of 185.
Mild Cognitive Impairment
Individuals with a childhood IQ (averaged across ages 7–13) in the range of 80–89 were considered to have mild cognitive impairment (N=120).
Neuropsychological Functioning
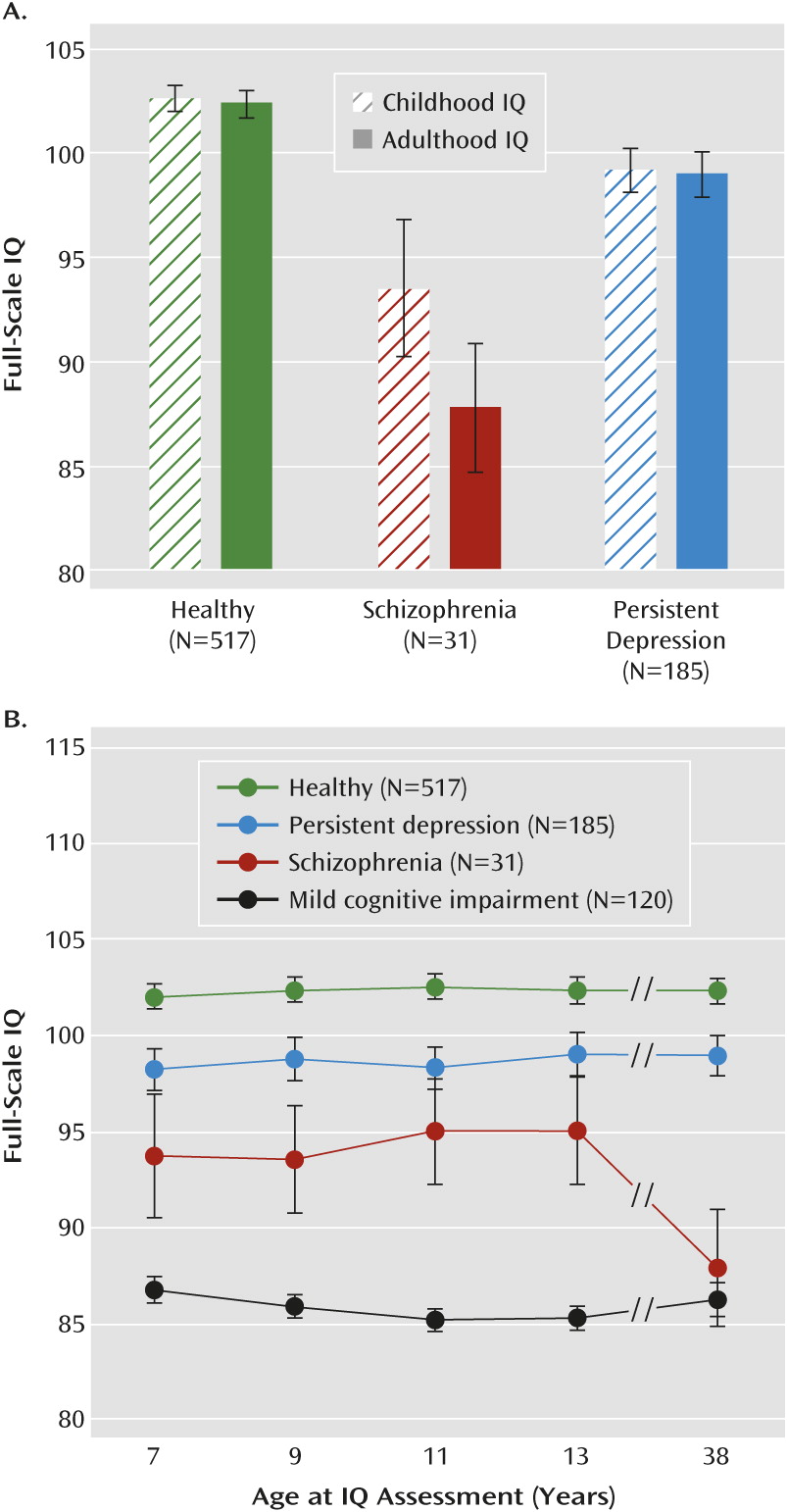
We assessed neuropsychological functioning using tests of IQ, learning and memory, processing speed, executive function, and motor function. Full-scale IQ can be thought of as an omnibus measure of general intellectual ability, because it captures overall ability across differentiable components of intellectual functioning (i.e., verbal IQ and performance IQ). Verbal and performance IQ can be further “unpacked” to make finer-grained distinctions in ability. Learning and memory, processing speed, executive function, and motor function represent even more basic mental functions.
IQ was assessed in childhood at ages 7, 9, 11 and 13 (before the onset of schizophrenia) and again in adulthood at age 38. We report a comparison of scores on the WISC-R (
40) and the WAIS-IV (
41). Full-scale, verbal, and performance IQ were standardized to population norms with a mean of 100 and a standard deviation of 15; subtest scaled scores were standardized to population norms with a mean of 10 and a standard deviation of 3. Learning and memory, processing speed, executive function, and motor function were each assessed at ages 13 and 38 using, respectively, the Rey Auditory Verbal Learning Test (
42), the Trail Making Test (
43), and the Grooved Pegboard Test (
42). (For details about each test, see Table S1 in the
data supplement that accompanies the online edition of this article.)
Informant-Reported Cognitive Problems
Informant reports of study members’ cognitive function were obtained at age 38. Study members nominated people “who knew them well.” These informants were mailed questionnaires and asked to complete a checklist, including whether the study member had problems with his or her attention and memory over the past year. The informant-reported attention problems scale consisted of four items: “Is easily distracted, gets sidetracked easily,” “Can’t concentrate, mind wanders,” “Tunes out instead of focusing,” and “Has difficulty organizing tasks that have many steps” (internal consistency reliability=0.79). The informant-reported memory problems scale consisted of three items: “Has problems with memory,” “Misplaces wallet, keys, eyeglasses, paperwork,” and “Forgets to do errands, return calls, pay bills” (internal consistency reliability=0.64). Informant-reported cognitive problems (attention and memory problems combined) were correlated with adult full-scale IQ (r=−0.22, p<0.0001).
Control Variables
DSM cannabis and alcohol dependence were assessed at ages 18, 21, 26, 32, and 38, and DSM hard drug (e.g., heroin, cocaine, amphetamines) dependence was assessed at ages 26, 32, and 38. Study members who were diagnosed with dependence at two or more assessments were considered persistently dependent on these substances.
Statistical Analysis
We compared the schizophrenia and persistent depression groups to a healthy group (a group of individuals in the cohort who had no current psychiatric disorder; N=518) on change in neuropsychological functioning from childhood to adulthood. Change scores were created by subtracting the childhood test score (averaged across ages 7–13 for the IQ tests and subtests) from the adulthood test score. Negative scores indicate neuropsychological decline. In
Tables 4 and
5, childhood and adulthood test scores as well as change scores are presented in standard deviation units (mean=0.00, SD=1.00). Standardized scores reflect effect sizes for how different each group is from the cohort norm. Differences between pairs of groups can also be interpreted as effect sizes. Effect sizes of 0.20, 0.50, and 0.80 reflect small, medium, and large effects, respectively (
44). Statistical tests involved planned orthogonal comparisons of each psychiatric group to the healthy group and were adjusted for sex, although results were unchanged when sex was excluded from the model.
Discussion
This study provides evidence of neuropsychological decline in schizophrenia from before to after illness onset in a population-based birth cohort of individuals followed prospectively from birth to age 38. This finding is consistent with previous longitudinal studies showing evidence of neuropsychological decline in schizophrenia (
11–
16,
18–
20).
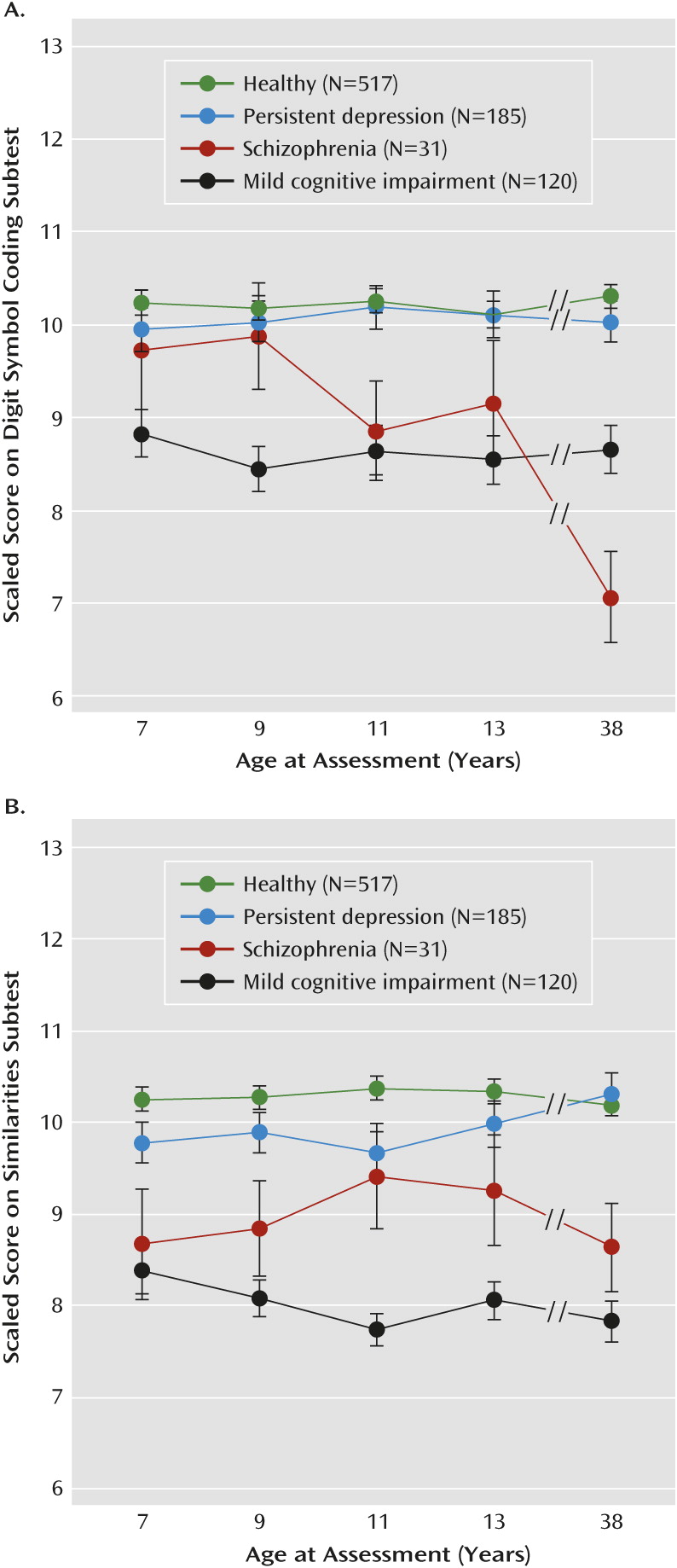
This study advances knowledge in several ways. First, previous longitudinal studies have focused almost exclusively on IQ. We showed that individuals with schizophrenia experienced declines in IQ as well as in a range of different mental functions, particularly those tapping processing speed, learning, executive function, and motor function. Decline was greatest on the digit symbol coding test, which is consistent with research suggesting that this test, more so than other neuropsychological tests, taps a core impairment in schizophrenia and may reflect network integration problems (
48). Decline was not ubiquitous across all mental functions, however. There was little evidence of decline in verbal IQ or delayed memory. Impaired verbal IQ and delayed memory among cohort adults diagnosed with schizophrenia could be traced back to childhood deficits that remained relatively stable across development. These findings highlight the importance of “unpacking” measures of generalized intellectual functioning, such as IQ, into more specific mental functions.
Second, we showed that neuropsychological decline was relatively specific to schizophrenia, as there was no evidence of decline among individuals in key comparison groups: children with mild cognitive impairment, at-risk children who did not develop schizophrenia, and individuals diagnosed with persistent depression. Research on the association between depression and neuropsychological decline has focused mainly on older adults and has yielded inconsistent findings, with some studies finding no association between depression and accelerated neuropsychological decline (
49,
50) and others finding a positive association (
51). Ours is the first study, to our knowledge, to examine depression-associated changes in neuropsychological functioning from before to after illness onset in a relatively young cohort. Notably, in our study, individuals with persistent depression performed worse than healthy individuals on a handful of neuropsychological tests in adulthood and were rated by informants as having more cognitive problems as adults than healthy individuals. Neuropsychological test deficits, however, were apparent from childhood, consistent with the interpretation that lower IQ constitutes a risk for depression (
52,
53).
Third, our findings suggest that neuropsychological decline in individuals with schizophrenia is nontrivial. Estimates of decline ranged from 1/3 to 3/4 of a standard deviation unit more than average for the healthy group on tests tapping processing speed, learning, attention, working memory, and motor function. Moreover, cognitive impairment in individuals diagnosed with schizophrenia was apparent in everyday life, as third-party informants noticed substantially more attention and memory problems in adults diagnosed with schizophrenia.
The results of this study should be viewed in the context of its limitations. First, although we found evidence of neuropsychological decline in schizophrenia from before to after illness onset, we could not fully map the developmental progression of neuropsychological deficits in schizophrenia from childhood to adulthood (funding agencies were unwilling to support repeated intellectual testing between ages 13 and 38). Nonetheless, we examined how deficits progressed from ages 7 to 13 for different mental functions and linked these deficits to data obtained at age 38. Deficits on the digit symbol coding test were not apparent at age 7 but increased gradually from ages 7 to 13, and by age 38, individuals with schizophrenia scored 1.08 standard deviations below the healthy group on this test. Notably, in an earlier report of the ages 7–13 neuropsychological test data (
26), we showed that children who would later develop schizophrenia exhibited slowed growth in performance on the digit symbol coding test, and based on this trajectory of slowed growth, we predicted the adulthood deficit of >1 standard deviation that we report here. This suggests that the “decline” in processing speed that we observed reflects a gradual, progressive process of slowed growth in this mental function that begins in childhood and continues beyond the early teen years. Exactly when in childhood the deficit in processing speed becomes evident is difficult to pinpoint, as at least two other studies have reported statistically significant deficits on the digit symbol coding test at approximately age 7 (
12,
54). Conversely, we showed that deficits on the similarities test emerged early but remained relatively static from age 7 through midlife. These findings imply that different pathophysiological mechanisms underlie the various neuropsychological deficits observed in schizophrenia patients.
A second limitation is that our findings are based on a relatively small group of individuals diagnosed with schizophrenia. The small group size prevented us from conducting an in-depth exploration of heterogeneity in neuropsychological decline. However, given reports of schizophrenia patients with IQs in the normal range (
55,
56) and the presumption that these patients have escaped neuropsychological decline, we asked if any of the individuals with schizophrenia in our cohort fit this profile. Of the 31 individuals diagnosed with schizophrenia, three individuals had both a childhood IQ of >100 and an IQ decline of <3.19 IQ points (the standard error of measurement of the WISC-R). While one of these three individuals performed in the normal range on all adult neuropsychological tests, each showed decline on the digit symbol coding test (mean=−0.88 standard deviations), suggesting that decline in processing speed is a core feature of schizophrenia. These findings further suggest that average to above-average neuropsychological test performance in a subset of adults diagnosed with schizophrenia cannot be used to infer that neuropsychological decline has not occurred. Rather, prospective baseline tests of neuropsychological functioning are necessary to document decline.
A third limitation concerns three unusual aspects of our sample that may limit the generalizability of our findings. First, the prevalence of schizophrenia is high. As we discussed earlier, this may be explained in part by a combination of our comprehensive repeated-measurement ascertainment strategy, high retention rates, and geographical variation. Second, a portion of individuals diagnosed with schizophrenia had not, to our knowledge, received treatment specifically for psychotic illness. These individuals had, however, come into contact with the mental health care system and were virtually indistinguishable on a variety of correlates from those who had been treated for psychotic illness. The one exception was that those who had not received treatment specifically for psychotic illness were from families of lower socioeconomic status, which might reflect an association between socioeconomic status and quality of care. Third, most individuals with schizophrenia were not taking antipsychotic medication in the year before adult testing. While this increases confidence that neuropsychological decline between ages 7–13 and age 38 is not due to recent antipsychotic medication use, it raises the question of whether our results are generalizable to patients currently taking antipsychotic medication. We noted very little difference in IQ decline between those who were (N=7; IQ decline, ∼7 IQ points) and those who were not (N=24; IQ decline, ∼6 points) taking antipsychotic medication in the year before testing. Bolstering the generalizability of our findings is the fact that our estimates of the IQ deficit in both childhood (9 points) and adulthood (15 points) precisely match estimates from meta-analyses of the premorbid IQ deficit in schizophrenia (
3) and the IQ deficit in first-episode schizophrenia (
1,
4).
This study has a number of implications. First, the results suggest that individuals diagnosed with schizophrenia experience neuropsychological decline from before to after illness onset. Second, however, the extent of decline and the developmental progression of decline varies considerably across mental functions that can be generally organized as fluid and crystallized abilities. Fluid abilities (e.g., processing speed, learning, executive function) showed the most substantial decline, with deficits in processing speed, for example, increasing gradually from childhood to beyond the early teen years. In contrast, crystallized abilities (e.g., verbal IQ) did not decline. Rather, these deficits were already apparent in childhood and remained static through midlife. This suggests that different pathophysiological mechanisms underlie the deficits in fluid and crystallized abilities seen in adult schizophrenia patients. Moreover, the findings highlight the fact that a substantial proportion of the neuropsychological deficits seen in adult schizophrenia patients is apparent before the onset of puberty, and future research on the emergence of neuropsychological deficits in schizophrenia should target early childhood to ascertain deficits in crystallized abilities and later childhood to ascertain deficits in fluid abilities. Finally, pharmacological and cognitive remediation therapies should target neuropsychological functioning as well as cognitive impairment in everyday life (
57), as treatment strategies that target both outcomes may have the greatest chances of success (
58).