Case Presentation
“Marie,” an 11-year-old African American girl of Caribbean descent with no previous psychiatric history, premorbid developmental impairments, or significant family psychiatric history, was admitted at the National Institute of Mental Health (NIMH) for participation in a longitudinal study of individuals with childhood-onset schizophrenia. She was reported to be a normally developing straight-A student who, not long before the onset of symptoms, had won a spelling bee. At age 10 years 3 months, after a brief trip to her father’s country of origin to attend a family funeral, she began to decompensate both emotionally and behaviorally. She had traveled to the region multiple times before, had not received any prophylactic medications or vaccines before the trip, and had no known sick contacts or exposures.
Over the next 13 months, Marie progressively deteriorated psychiatrically. Through a detailed review of the records, discussions with her outpatient providers, and multiple interviews with both parents, it was found that the patient’s initial presentation was thought to be attributable to a major depressive episode with psychotic features, particularly given the context of the death of a close relative. Marie exhibited diminished emotional expression, avolition, alogia, and asociality. She subsequently received multiple antidepressant treatment trials, which were ineffective as she continued to deteriorate. Soon after her initial symptoms arose, she began to discuss paranoia and delusions and respond to internal stimuli, and she became increasingly disorganized. Antipsychotics were added to her regimen, without appreciable benefit.
Although Marie’s symptoms did not fit clearly into a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) (1), her providers, struggling with the severity and character of her symptoms, administered several trials of antibiotics, again with no effect. Over the following several months, she had numerous inpatient psychiatric admissions for physical aggression and psychosis, the longest of which lasted 3 months. During her admissions and the periods that followed, combinations of antidepressants, maximally dosed, and antipsychotics, minimally dosed, were administered with no effect.
MRI and lumbar puncture were performed. The MRI was unremarkable. Results of the lumbar puncture suggested the possibility of an elevated intracranial pressure; the records were unclear, and on direct communication the providers could recall no further specifics. A repeat lumbar puncture was performed, and the results were normal. Nevertheless, apparently because of the patient’s lack of response to other treatments, she had received treatment with acetazolamide, which made no difference to her symptoms. Lumbar puncture was repeated at NIMH several months after the discontinuation of the acetazolamide and while the patient was symptomatic, and the results were again within normal limits.
Subsequently, Marie’s providers discontinued antipsychotic and antidepressant prescriptions, instead providing as-need doses of clonazepam and directing her to emergency departments for agitation whenever her parents could not manage, and they referred her to NIMH for participation in the childhood-onset schizophrenia study.
Since 1991, patients meeting DSM-IV criteria for schizophrenia with an onset of psychosis before age 13 have been recruited nationally for participation in a longitudinal study of individuals with childhood-onset schizophrenia conducted at NIMH. The protocol includes screening, evaluation, diagnosis, treatment optimization, and follow-up phases. Patients and first-degree relatives are interviewed over a period of 3 days for current and lifetime psychiatric disorders. Extended inpatient evaluation lasts up to 4 months and includes initial observation, medication taper, medication-free observation, and stabilization/treatment optimization phases. Before a definitive diagnosis is made, medical causes for a patient’s presentation are aggressively explored, often with the aid of consulting specialties, and without limitation. Once diagnosed, the patient enters the treatment optimization phase and is given appropriate clinical treatment until stabilized. Longitudinal outpatient follow-ups are offered every 2 years for subsequent evaluation, and more frequently if indicated (2).
Marie was screened by the childhood-onset schizophrenia team at NIMH. The patient and her parents were interviewed individually and as a group over a 3-day period using both clinical and structured interviews. The patient was a well-nourished, well-developed African American female who appeared her stated age. She was dressed in personal attire, which was stained with food from breakfast. She had fair hygiene with poor grooming. She sat in a wheelchair (an environmental measure her parents used to cue her to the need to remain seated) speaking unintelligibly to herself and occasionally lashing out aggressively, without any identifiable trigger, toward anyone sitting next to her. She did not make eye contact and was constantly looking around the room as though she was tracking something that could not be seen by others. She was not cooperative with the interview, was not engageable, and would not respond to questions directly or appropriately. She did not have any abnormal movements or tics. Her speech was minimal, with a regular rate and rhythm, but consisted primarily of neologisms and was delivered at a high pitch. She frequently spontaneously uttered foul language and wrote profane statements interspersed with unintelligible scribbling on paper. Her affect spanned the range from smiling and laughing to herself to sitting calmly to violently lashing out. It was labile, unstable, and inappropriate. Her thought process was disorganized, and because of the degree of disorganization, the constant responding to internal stimuli, and the difficulty of engaging the patient directly, her thought content could not be assessed at the time of the screening. (It was later determined to be significant for paranoid delusions.) The patient was awake and alert, but oriented only to self. Her insight and judgment were impaired/poor.
Marie was admitted to the inpatient unit for further observation and workup, with a working diagnosis of psychosis not otherwise specified. Her medications at screening included clonazepam, 0.5 mg/day as needed (administered once in the 14 days prior to her admission), melatonin, 1.5 mg at bedtime, acetazolamide, 250 mg/day, and botanical preparations to aid sleep and reduce anxiety (Calms Forté Kids, once daily, and Rescue Remedy, four drops daily). During her initial 24 hours on the unit, the patient was noted to be actively hallucinating, responding to internal stimuli, expressing paranoid delusions, and exhibiting significant periods of disorganized speech and behavior, and she was physically agitated and aggressive. The team continued to obtain collateral information, including a comprehensive review of records, from all of her contacts with medical and mental health providers, and communicated directly with her previous providers. Given the patient’s presentation and history, a diagnosis of childhood-onset schizophrenia was made. Her medications were discontinued and clozapine therapy was initiated.
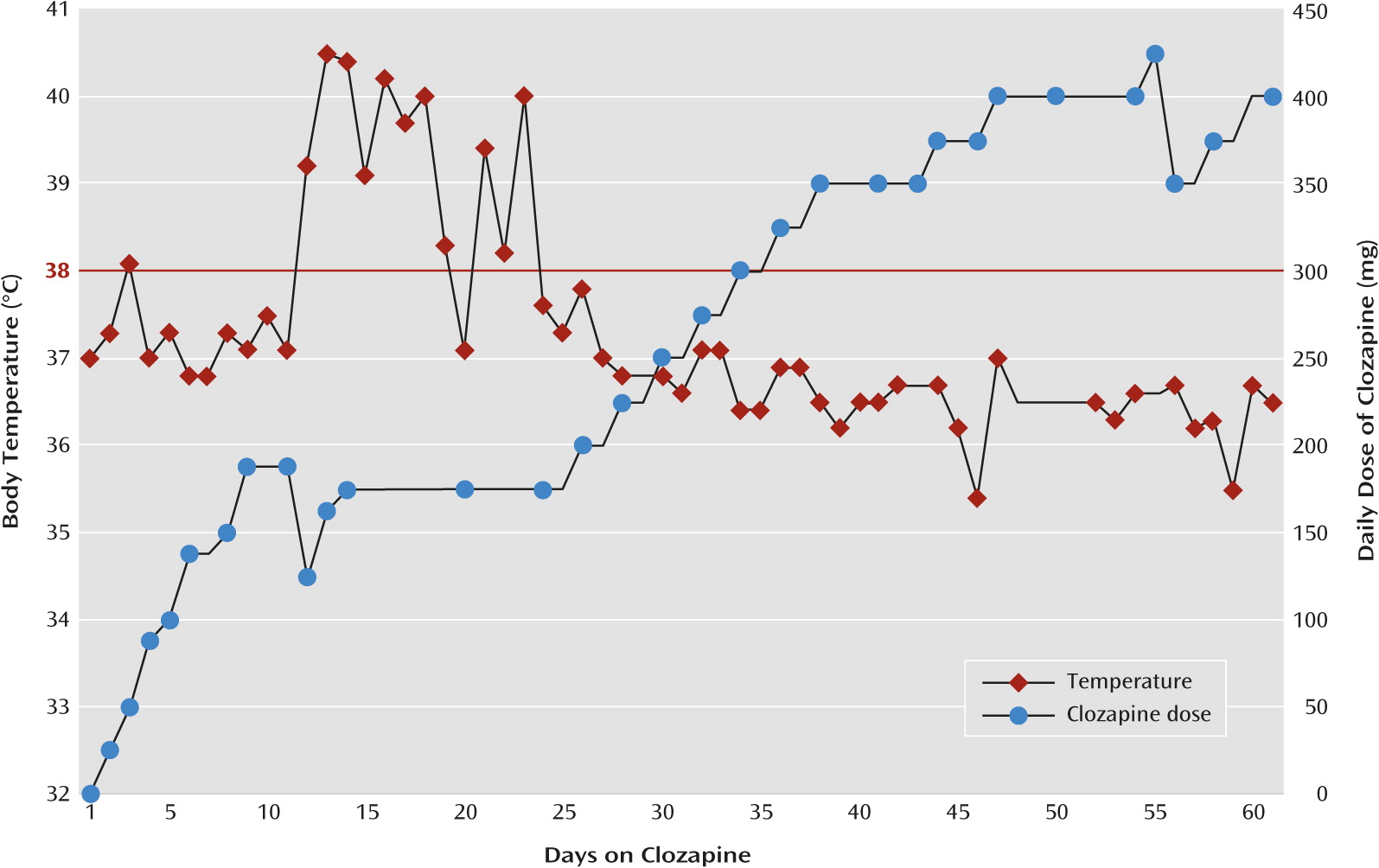
The clozapine dosage was rapidly titrated over the next 7 days to a total daily dose of 175 mg. On day 2 of the titration, at a daily dose of 50 mg, the patient developed a fever (defined as an oral temperature ≥38°C), which peaked at 38.1°C as verified by measurement of vital signs every 8 hours, and spontaneously remitted after 24 hours. The patient began to show gradual but significant functional and symptomatic improvement over the next 10 days; she reported less paranoia and delusions, became increasingly organized, began to attend to her hygiene and appearance, reported a decrease in her hallucinations, was redirectable, was less agitated and aggressive, and was increasingly interactive, even participating in brief educational activities. However, the fever returned on day 11, four days after her clozapine dosage had reached 175 mg/day. The fever vacillated over the next 12 days, peaking at 40.5°C in the context of both acetaminophen use, ranging from 0–1950 mg within a 24-hour period, and other supportive measures, such as cold compresses and hydration. During this period, the clozapine dosage was not adjusted (Figure 1).
Because no cause was identified for the fever, no other treatments were initiated. Probable etiologies were aggressively explored, with multidisciplinary consultations from the pediatrics, infectious diseases, genetics, neurology, obstetrics and gynecology, rheumatology, ophthalmology, and endocrinology departments. An extensive workup ensued, which included evaluations for epidemiologic, vascular, infectious, inflammatory, autoimmune, metabolic, neoplastic, congenital, degenerative, and endocrine causes for Marie’s fever as well as her psychiatric presentation. Abnormal laboratory test results during the workup included an elevated creatine kinase level of 749 IU/L (reference range, 0–143) on hospital day 6; an elevated white blood cell (WBC) count of 13.43×103/mm3 with eosinophilia (9% with an absolute count of 1.21×103/mm3) on hospital day 21; and an elevated WBC count of 19.59×103/mm3 with neutrophilia (83.6% with an absolute count of 16.38×103/mm3) and mild eosinophilia (3.1% with an absolute count of 0.61×103/mm3) on hospital day 23.
On hospital day 24, the patient’s temperature returned to normal and the leukocytosis and eosinophilia remitted. As all other laboratory and imaging test results had been unremarkable, the team concluded unanimously that the fever was secondary to the clozapine use. Monitoring of the patient continued, and all repeat laboratory tests remained normal. The fever did not recur over the next 8 weeks of her hospital stay. At discharge, the patient reported ongoing but decreased paranoid delusions, had become organized, was fully oriented, was attending to her hygiene and appearance, reported ongoing but decreased auditory and visual hallucinations, was less agitated and aggressive, was increasingly interactive, was engaged with staff and peers, displayed a full range of affect, and was appropriately interactive with her peers on the unit. Her serum level of N-desmethylclozapine (NDMC, an active metabolite of clozapine), level was 258 ng/mL, and her serum clozapine level was 681 ng/mL, resulting in an NDMC/clozapine ratio of 0.38 (3, 4).
Six months after discharge, the patient’s fever had not recurred and her parents reported that she continued to show progressive improvement in symptoms, cognition, and functioning and had even returned to school and was attending classes, with support, for 6 hours daily.
Discussion
Clozapine has demonstrated superior efficacy in the treatment of refractory schizophrenia. It is well recognized as the treatment of choice in adults, and its superiority over other antipsychotic drugs has been established for over 20 years (
5,
6). Although its use in children and adolescents is a relatively recent phenomenon, there are clear data supporting its superior efficacy in these groups as well (
5,
7–
14). Clozapine remains a third-line medication, however, primarily because of its side effect profile. Moreover, clinicians generally shy away from the use of clozapine, even as a third-line drug, as evidenced by epidemiological studies demonstrating that its use occurs much later than recommended by the clinical guidelines (
7,
15).
The use of clozapine as a last-resort drug makes the management of adverse events particularly challenging, as the common strategies of switching or tapering are generally not an option. Although most of the side effects of clozapine can be managed by augmentation strategies (
7), malignant hyperthermia, neutropenia, agranulocytosis, and neuroleptic malignant syndrome, all of which may involve a fever, are potentially life threatening and thus present particular challenges. Clear guidance is available for the management of neutropenia, agranulocytosis, and neuroleptic malignant syndrome, but much less is known about the management of clozapine-induced fever, which in adults is a frequent complication, with an incidence ranging from 2% to 55% (
14,
16–
23). The relevance of establishing a guideline is highlighted by our observation of an increase in the number of children referred to our study for whom clozapine has been prescribed.
To our knowledge, this is the first report of the management of clozapine-induced fever in a child. This patient’s premorbid course and presentation, although not typical, are consistent with a diagnosis of childhood-onset schizophrenia. This patient developed a persistent fever, and eventually a brief leukocytosis, for which no other identifiable cause could be determined. In addition, her symptoms remitted spontaneously without intervention and have not returned to date despite subsequent further titration of the clozapine (
Figure 1). Although the fever lasted much longer than the previously reported average 1- to 9-day period (
19), it was characteristically consistent with the literature (
Table 1), and after carefully ruling out other potential causes, the final determination for the etiology of this patients’ fever was that it was induced by clozapine.
Several important elements of the differential diagnosis should be carefully ruled out when managing a patient who presents with a fever in the context of clozapine use. Neuroleptic malignant syndrome, agranulocytosis, neutropenia, and malignant hyperthermia are life threatening and should be considered immediately (
24,
25).
Table 2 summarizes several key differences in the clinical presentation and laboratory abnormalities seen in each. It should be noted that although an elevated creatine kinase level has become synonymous with neuroleptic malignant syndrome, we often see elevated values up to 800 U/L with psychotic agitation. Clozapine-induced neutropenia and agranulocytosis are more common in children than in adults and usually occur 1–3 months after starting the drug (
26). When these side effects occur, fever is most often the cardinal sign. However, unlike in a case of a drug-induced fever, a thorough investigation will quickly reveal that the fever has an infectious etiology that has occurred because of the loss of white blood cells (
27).
The exact mechanism for clozapine-induced fever is unknown (
19). Prevailing theories include a mild variant of neuroleptic malignant syndrome, an allergic reaction, and the immunomodulating effects of the drug (
16). The latter two theories are supported in the literature by several case reports and a prospective study, which have reported eosinophilia and elevated levels of cytokines, particularly interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), above what is already expected in schizophrenia, in the context of clozapine-induced fever (
17,
28–
35). Additionally, clozapine has been demonstrated to have direct effects on these markers in adult patients with schizophrenia (
28,
29,
32).
IL-6 and TNF-α are involved in systemic inflammation and are members of a group of cytokines that, in addition to numerous systemic processes, stimulate the acute phase reaction, regulate immune cells, induce apoptotic cell death, and mediate fever (
28). These cytokines exert their fever-modulating effects by elevating the set point of body core temperature via temperature-sensitive neurons in the preoptic area of the hypothalamus (
36). Interestingly, the mRNA expression of these inflammatory markers has also been noted to be increased in the dorsolateral prefrontal cortex of schizophrenia patients, suggesting that therapies aimed at immune system attenuation might have direct beneficial effects in the brain in schizophrenia (
37).
With the detection of fever, a common initial response on the part of a physician would be to withhold or discontinue the clozapine. However, this can trigger a considerable psychotic decompensation in an already extremely ill patient. In the case of the patient described in the vignette, as is often the situation, clozapine was a last-resort medication. Given the relatively benign nature of the fever, it is not in the best interest of the patient to automatically discontinue the medication. It is important to carefully rule out life-threatening causes of the fever while continuing the use of clozapine with heightened monitoring. Once other causes have been ruled out, continuing the use of clozapine while providing supportive care for the fever is the best course of action.