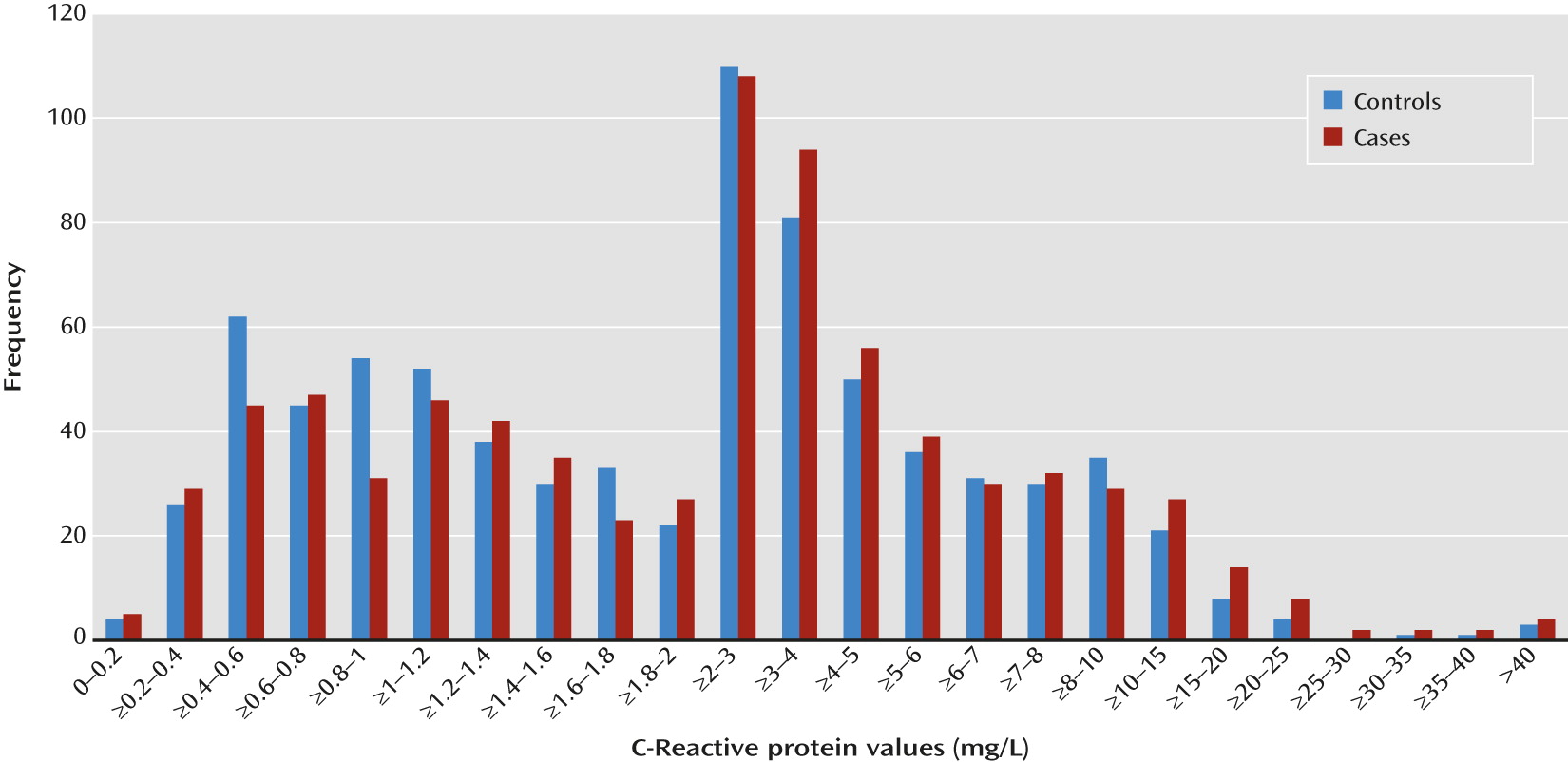
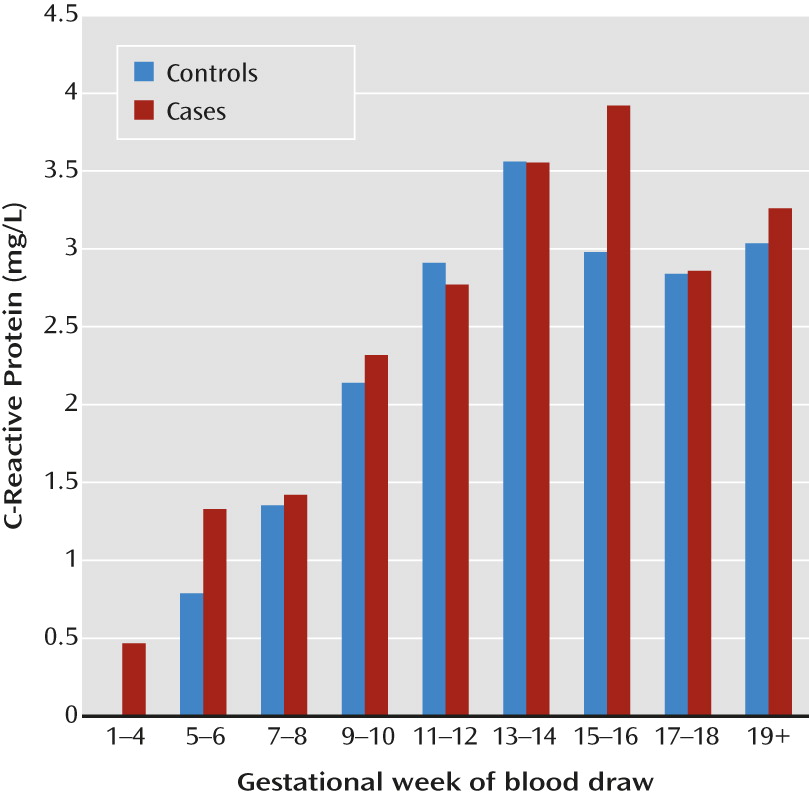
Mounting epidemiological and preclinical evidence implicates prenatal infection and subsequent immune activation in the etiology of schizophrenia (for a review, see reference
1). The most convincing epidemiologic studies were based on birth cohorts in which maternal biomarkers of infection and inflammation were assayed from prospectively archived maternal serologic specimens drawn during pregnancy. These studies revealed associations between offspring with schizophrenia and elevated maternal antibody to influenza, rubella, toxoplasma gondii, and herpes simplex virus type 2 (
1–
6). Associations have also been found using ecologic designs and ascertainment of pregnancies complicated by clinical infections (
7–
11). Inflammation during pregnancy may represent a common pathway by which different infections, as well as other early environmental insults, increase risk for the disorder. In support of this, other birth cohort studies found that levels of two proinflammatory cytokines, interleukin-8 and tumor necrosis factor-alpha, were significantly elevated in maternal serum samples from pregnancies that gave rise to offspring who later developed schizophrenia (
12,
13). Moreover, many autoimmune diseases that result in a chronic inflammatory state have been found to be associated with schizophrenia (
14). Risk of schizophrenia was found to be further increased in persons with autoimmune diseases that also experienced a severe infection, suggesting that multiple perturbations that produce inflammation may act synergistically (
14).
Method
The Finnish Prenatal Study of Schizophrenia is based on a nested case-control design. This study is part of a larger program of research known as the Finnish Prenatal Studies, aimed to examine prenatal exposures in relation to major psychiatric outcomes, including schizophrenia and autism. The sampling frame was defined so that all members of the cohort were within the age of risk for schizophrenia. For this purpose, the sampling frame consisted of all offspring born in Finland from 1983 (the beginning of the Finnish Maternity Cohort) to 1998. Cohort members were followed up until 2009.
Description of the Cohort and Biobank
All offspring in the Finnish Prenatal Study of Schizophrenia were derived from the Finnish Maternity Cohort, which consists of virtually all pregnancies with archived prenatal serum specimens that were drawn beginning in 1983. Sera were drawn during the first and early second trimesters from more than 98% of pregnant women in Finland, following informed consent, for screening of HIV, syphilis, and hepatitis. One maternal serum sample was obtained for each pregnancy. Over the years of births in the study, sera from over one million pregnancies were drawn. After the screening, serum samples were stored as one aliquot at −25°C in a single, centralized biorepository at the National Institute for Health and Welfare in Oulu, Finland. All of the serum samples in the Finnish Maternity Cohort can be linked with offspring by a unique personal identification number, which has been assigned to all residents of Finland since 1971.
Case and Control Identification
In order to identify cases for the present study, we utilized the nationwide Finnish Hospital Discharge and Outpatient Registry. The Finnish Hospital Discharge and Outpatient Registry contains all recorded diagnoses for all psychiatric hospital and outpatient admissions. The registry was established in 1963; computerized data are available from 1987 to the present. All Finnish citizens are entitled to Finland’s national health insurance, which is maintained by the state and financed through tax revenues. The registry covers all mental and general hospitals, as well as all inpatient wards of local health centers, military wards, prison hospitals, and private hospitals. The registry contains the hospital identification code, the dates and length of stay, and the primary diagnoses at discharge. Individuals in the Finnish Maternity Cohort with a diagnosis of schizophrenia (ICD-10 F20) or schizoaffective disorder (ICD-10 F25) were followed up from 1998 to 2009 (hereafter, these cases are referred to as “schizophrenia” cases). All diagnoses were made in accordance with ICD-10. The age at onset was dated by the first recorded contact with a psychiatric facility with a diagnosis of schizophrenia or schizoaffective disorder. Diagnostic validity of schizophrenia based on the Finnish Hospital Discharge and Outpatient Registry was found to be excellent; in a previous validation study (
19), 93% of patients with a Finnish registry diagnosis of schizophrenia were assigned a consensus diagnosis of schizophrenia or schizophrenia spectrum disorder. These cases were linked to the Finnish Maternity Cohort by the personal identification numbers in order to identify the corresponding maternal serum specimens. For the present study, we identified a total of 1,514 cases of schizophrenia. Among these schizophrenia cases, 777 (schizophrenia, N=630; schizoaffective disorder, N=147) had sufficient maternal sera available to perform C-reactive protein testing. These cases were matched 1:1 to controls drawn from the birth cohort who were without schizophrenia, other nonaffective psychotic disorders, and bipolar disorder based on date of birth (within 1 month), sex, and residence in Finland at the time of diagnosis.
Finnish Population Registry
The computerized Finnish Population Registry was created in 1971 when the nationwide centralized population registry was established. The registry contains comprehensive data on place of birth, twin/singleton birth, date of emigration, date of death, place of residence, and biological parents, including their birth dates.
The study was approved by the ethical committees of the hospital district of Southwest Finland, the National Institute for Health and Welfare (which also included register linkage approval), and the institutional review board of the New York State Psychiatric Institute. Informed consent was obtained before acquisition of all maternal serum specimens and following explanation of the nature and possible consequences of the procedure and data derived from serum analyses.
C-Reactive Protein Assay
C-reactive protein measurements were carried out blind to case/control status. C-reactive protein was measured on the clinical chemistry analyzer Architect c8200 (Abbott Laboratories, Abbott Park, Ill.) using a latex immunoassay (Sentinel, Milan, Italy) at the National Institute for Health and Welfare laboratory in Helsinki, Finland, under the supervision of Dr. Leiviskä. During the course of the study, the precision between series expressed as the coefficient of variation was 5.1% (SD=2.3%), and the systematic error (bias) was 2.7% (SD=7.4). Assay sensitivity was 0.10 mg/L.
Covariates
The covariates included maternal age; paternal age; number of previous births; socioeconomic status (based on maternal education); maternal and parental history of schizophrenia, other nonaffective psychotic disorders, and affective or other psychiatric disorders (a list of ICD codes used are presented in the
Table 1 footnote); gestational week of the maternal blood draw; twin/singleton birth; urban/semiurban/rural birth; and province at birth. All covariates except gestational week of the blood draw were obtained from the Finnish Population Registry; gestational week was obtained from the Finnish Maternity Cohort. Each covariate was classified as presented in
Tables 1 and
2.
Statistical Analysis
The analysis was based on a nested case-control design in which the control subjects for each case were selected from the population at risk (the Finnish Prenatal Study of Schizophrenia birth cohort) and matched to cases based on selected factors, as described in “Case and Control Identification.” In the main analysis, we examined maternal C-reactive protein as a continuous measure. Given the skewed distribution of C-reactive protein, the variable was log-transformed before analysis.
In order to further facilitate interpretation of the data, we conducted an additional analysis with maternal C-reactive protein as a categorical variable. C-reactive protein levels ≥10 mg/L are considered clinically abnormal (
18). Therefore, we examined the risk of developing schizophrenia among offspring of mothers with C-reactive protein levels ≥10 mg/L in relation to those with levels <10 mg/L.
Appropriate to the nested case-control study design, point and interval estimates of odds ratios were obtained by fitting conditional logistic regression models for matched sets. Statistical significance was judged at a p value <0.05. After examining the main effects, we then investigated whether the effect of maternal C-reactive protein on schizophrenia risk was modified by sex. For this purpose, sex and sex-by-C-reactive protein interaction terms were added to the statistical model. The interaction terms were deemed to be statistically significant based on a p value <0.05. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, N.C.).
Discussion
This study demonstrated that elevated maternal C-reactive protein during pregnancy is associated with an increased risk of schizophrenia in offspring. This finding was not confounded by maternal age, previous births, maternal education, parental psychiatric disorders, urbanicity of birth, province of birth, twin/singleton birth, or gestational week of the blood draw. Given the design advantages, this study provides the most robust evidence to date that maternal inflammation during pregnancy is related to the risk of schizophrenia in offspring and is consistent with many preclinical studies that have suggested a causal association.
There are several biologically plausible hypotheses to account for this association. First, C-reactive protein may be acting as a proxy for the inflammatory cytokine, interleukin-6 (
20). Preclinical studies indicate that interleukin-6 may mediate some of the effects of maternal immune activation on schizophrenia-related behavioral phenotypes in rodents (
21–
23). Following maternal injection of polyinosinic-polycytidylic acid or lipopolysaccharide, levels of several proinflammatory cytokines, including interleukin-6, are elevated in the maternal serum, the placenta, and possibly the brain of the developing offspring, although the latter remains controversial (for a review, see reference
17). Indeed, Smith et al. (
23) elegantly demonstrated that the proinflammatory cytokine interleukin-6 is both necessary and sufficient to produce the behavioral abnormalities in sensorimotor gating and latent inhibition observed in adult offspring of mothers given an injection of polyinosinic-polycytidylic acid during pregnancy. We were not able to quantify interleukin-6 in the present study given the sample volume requirements for this assay. In addition to acting as a proxy for interleukin-6, C-reactive protein may also affect fetal development through certain mediating factors. Clinical studies have shown that increased C-reactive protein in pregnant women is associated with conditions such as preeclampsia as well as with preterm birth and lower birth weight (
24–
26), each of which is related to adverse early or later reproductive outcomes. In fact, it has been hypothesized that C-reactive protein itself may contribute to placental dysfunction in preeclampsia by eliciting endothelial dysfunction, resulting in vascular damage and impaired placental development (
27,
28). Finally, C-reactive protein may directly affect brain development. It is involved in the complement cascade, activation of which is known to play a role in normal synaptic pruning and refinement during development (
29). If elevated levels of maternal C-reactive protein result in increased levels of C-reactive protein in the developing offspring brain, it could conceivably alter synaptic connectivity in a way that increases risk for the development of psychopathology.
Although to our knowledge no previous study has examined maternal C-reactive protein in relation to schizophrenia in offspring, maternal cytokines have been investigated in two previous birth cohort studies. Maternal tumor necrosis factor-alpha levels were related to a significantly increased risk of schizophrenia and other psychotic disorders (N=27) in offspring in the National Collaborative Perinatal Project (
13). Significantly elevated maternal interleukin-8 levels were related to an elevated risk of schizophrenia and other schizophrenia spectrum disorders (consisting mostly of schizoaffective disorder) (N=59) in the Child Health and Development Study birth cohort (
12).
Strengths of our study included C-reactive protein levels from prospectively drawn archived maternal serum specimens. Moreover, the specimens were drawn during early to middle pregnancy, rather than at delivery or in the neonate, allowing for a greater focus on prenatal influences. In addition, case ascertainment was facilitated by the socialized health care system of Finland, which covers all individuals who seek treatment for schizophrenia, encoded in national psychiatric registries. This allowed us to obtain nearly all schizophrenia cases diagnosed in Finland in a national population-based birth cohort. In addition, the Finnish Population Registry permitted the identification of control subjects who are representative of the source population that gives rise to the cases. These methodological features minimize the potential for selection bias.
There are multiple exposures that could elevate levels of maternal serum C-reactive protein, including both infectious and noninfectious insults. The fact that risk of developing schizophrenia was elevated in offspring of mothers with C-reactive protein levels ≥10 mg/L (compared with offspring of mothers with levels <10 mg/L) suggests that elevated C-reactive protein may reflect a recent infection or an active inflammatory process, since levels ≥10 mg/L are considered clinically abnormal (
18). This interpretation is consistent with an extensive literature documenting an increased risk of schizophrenia among offspring whose mothers experienced an infection during pregnancy (for a review, see reference
1). However, the fact that we also found a significant increase in risk for schizophrenia for C-reactive protein levels expressed as a continuous variable (following log transformation) may indicate that even mildly elevated levels, which may reflect a low-grade inflammatory process, are related to schizophrenia in offspring. In either case, it seems likely that elevated levels of maternal C-reactive protein, and the underlying immunological activation these levels likely reflect, interact with other environmental insults to give rise to this disorder. This hypothesis is supported by a recent preclinical study in rodents demonstrating that mild maternal immune activation during pregnancy interacted with subsequent peripubertal stress in offspring to give rise to behavioral abnormalities in adulthood considered to be analogous to those found in schizophrenia (
30). Alternatively, elevated maternal C-reactive protein levels may reflect genetic, rather than environmental, factors. Several common polymorphisms in the C-reactive protein gene have been associated with elevated levels of serum C-reactive protein (
31). Future work will be necessary to assess interactions between maternal infection, peripubertal stress, and other postnatal environmental risk factors, as well as with genetic susceptibility, in schizophrenia.
There are several limitations to this study. First, although there was no evidence of confounding following extensive testing of many covariates, residual confounding by unmeasured factors may have occurred. Second, because the members of the sample cohort were born in 1983 and the last follow-up year was 2009, our sample was relatively young, with the mean age for both case and control subjects being 22.8 years (SD=2.2) and the mean age at first treatment for case subjects being 19.0 years (SD=2.7 years). Thus, it is possible that C-reactive protein is a risk factor for earlier-onset cases of schizophrenia. Additionally, C-reactive protein may be a risk modifier rather than a risk factor for schizophrenia; in this scenario, elevated levels would be related to an earlier onset of the disease in persons at risk for other reasons.
Although the primary goal of our analysis was to investigate an association between maternal C-reactive protein and later risk of schizophrenia in offspring, in the course of testing for confounding, we demonstrated associations in this cohort between schizophrenia and family psychiatric history, twin/singleton birth, urbanicity, and province of birth (
Table 2). The increased risk of schizophrenia in subjects with a parent with either a schizophrenia spectrum disorder or nonaffective disorder or a parent with any type of psychiatric diagnosis was expected given the findings reported in the previous literature. Specifically, it has been demonstrated that having a mother or a parent with schizophrenia increases the offspring risk of schizophrenia by six- to eightfold (
32,
33). Our finding of a decreased risk of schizophrenia in twin, compared with singleton, pregnancies differs from the findings of a previous study in a Danish sample, which showed that twin offspring had a 26% increased risk of schizophrenia compared with singleton offspring (
34). Another study, conducted in a West African population, showed that twinning had no relationship to schizophrenia risk (
35). Potential explanations for these varying results include an interaction of this variable with genetic background or mediation by levels of prenatal care and obstetric complications, which could vary by the country of birth. The finding in our study of an association between urbanicity and schizophrenia has also been demonstrated in previous epidemiologic studies (
33,
36). While the reasons for this association are unknown, hypothesized mediators include increased risk of infections or other factors related to urbanicity, selective migration, and increased access to psychiatric services (
37). Finally, our finding that living in the Southern Province of Finland increased the risk of developing schizophrenia was unexpected. In fact, a previous study reported that the prevalence of psychoses was highest in Northern and Eastern Finland (
38), and thus understanding the origin of our finding will require further investigation.
While the covariates were associated with increased risk of schizophrenia, they did not confound the relationship between maternal C-reactive protein and schizophrenia because none of them were independently related to levels of maternal C-reactive protein (
Table 1) and the association between maternal C-reactive protein and schizophrenia remained significant after adjusting for all of these covariates. However, it is still possible that these covariates may be effect modifiers of the relationship between maternal C-reactive protein and schizophrenia. For example, maternal C-reactive protein may interact with familial risk or urbanicity to increase risk of this disorder. Although outside the scope of the present study, we aim to explore these questions in future work.
Moreover, elevated maternal C-reactive protein levels may not be a risk factor that is specific to schizophrenia.
In the same Finnish national birth cohort that was investigated in this study, our research group previously demonstrated a significant increase in maternal C-reactive protein levels in pregnancies that gave rise to childhood autism (autistic disorder) (
39). In support of an early inflammatory contribution to the pathophysiology of autism, several other studies have found evidence that severe maternal infection during pregnancy or elevated levels of inflammatory signaling molecules in the amniotic fluid or neonate are associated with an increased risk of autism (
40–
43), although there are also negative reports (
44,
45). While we have not yet investigated maternal C-reactive protein and bipolar disorder or major affective disorder in the present cohort, there is evidence that maternal infection is a risk factor for development of these disorders in offspring, suggesting that an inflammatory component may also play a role in these outcomes (
46–
48).
It is intriguing to speculate that maternal inflammation during pregnancy may “prime” the brain to broadly increase risk for the later development of different types of psychiatric syndromes. This is consistent with preclinical studies that have demonstrated that maternal immune activation during pregnancy produces offspring with behavioral and brain phenotypes that are most likely relevant to multiple psychiatric disorders, especially schizophrenia and autism (
15,
17). Interaction with specific genetic or environmental insults, during particular developmental windows, might then determine the specificity of the later disorder. This hypothesis could be tested in future studies.
In conclusion, we have demonstrated that elevated maternal C-reactive protein is related to an increased risk of schizophrenia in offspring. These findings are consistent with an extensive preclinical literature on maternal immune activation and brain and behavioral abnormalities in animal models, as well as with epidemiologic evidence of maternal infection and immunologic dysfunction as risk factors for schizophrenia. If replicated, these findings may have important implications for elaborating the role of immune system dysfunction in schizophrenia. Finally, our finding that maternal C-reactive protein is associated with an increased risk of schizophrenia in offspring has important implications for disease prevention, given that many standard approaches already exist to reduce the incidence of infections or lessen the severity of the inflammation that they produce (
49).