Alpha7 nicotinic acetylcholine receptor (α7 nAChR) is encoded by
CHRNA7 located at 15q13-q14. This region has been reported to be associated with schizophrenia and a deficit in the P50 waveform of the auditory event-related potential (
1,
2). Homomeric α7 nAChR, which consists of five α7 subunits, has low affinity for nicotine and its endogenous ligand, acetylcholine (
3). α7 nAChRs are widely distributed in the brain (
4,
5) and especially highly expressed in the prefrontal cortex and hippocampus (
6,
7), two regions involved in a number of cognitive functions. α7 nAChRs mainly act presynaptically in the brain (
8), although they exist at both pre- and postsynaptic sites (
9,
10). At presynaptic sites, activation of α7 nAChRs leads to increased permeability to cations, including Ca(2+), and facilitates neurotransmitter release, including the release of noradrenaline, serotonin, GABA, glutamate, and dopamine (
3,
11). Postsynaptically, α7 nAChRs are implicated in phosphorylation and regulation of gene expression (
10,
12).
CHRNA7 has a partial duplication that constitutes the α7-like nicotinic receptor gene,
CHRFAM7A (see Figure S1 in the
data supplement accompanying the online version of this article), which is unique to humans (
13) and expressed in human brains at approximately 10-fold lower levels than
CHRNA7 (
11). This chimeric gene,
CHRFAM7A, is considered to be a dominant negative regulator of function when coexpressed with the
CHRNA7 gene product, α7 nAChR (
11), because it lacks the majority of the neurotransmitter-gated ion-channel ligand binding domain but retains the transmembrane region that forms the ion channel.
Genetic studies of
CHRNA7 and
CHRFAM7A identified several schizophrenia-related single-nucleotide polymorphisms (SNPs), haplotypes, and mutations (
2,
14–
17). There were also reports of associations of single SNPs and haplotypes with bipolar disorder and major depression (
18,
19). A number of postmortem brain studies of α7 nAChRs in schizophrenia, using [
125I]- α-bungarotoxin, for which α7 nAChRs have high affinity, indicated that subjects with schizophrenia have decreased binding of [
125I]- α-bungarotoxin in various brain regions, including the frontal cortex and hippocampus (
20,
21). In bipolar disorder, bungarotoxin binding in the CA1 and perirhinal cortex was increased (
22). In contrast to these rather consistent results reflecting α7 nAChR binding abilities in schizophrenia, the results on
CHRNA7 transcript levels were fewer and less clear (
23). The mRNA levels of
CHRNA7 and
CHRFAM7A in the dorsolateral prefrontal cortex from subjects with schizophrenia and bipolar disorder were not significantly different from comparison subjects (
24), and in the hippocampus,
CHRNA7 mRNA levels were significantly decreased in nonsmokers with schizophrenia compared with comparison nonsmokers (
25). In addition, two postmortem studies examined the effect of smoking on expression of
CHRNA7 (
25,
26). In the hippocampus of patients with schizophrenia, both
CHRNA7 mRNA and protein were elevated in smokers compared with nonsmokers (
25), and in the temporal cortex of comparison subjects,
CHRNA7 protein levels were also higher in smokers than in nonsmokers (
26). The incidence of smoking in schizophrenia is very high (more than 80% of schizophrenia patients smoke compared with 25% of the general population [
27]), and thus smoking constitutes a potential confounding factor in the studies of schizophrenia.
Discussion
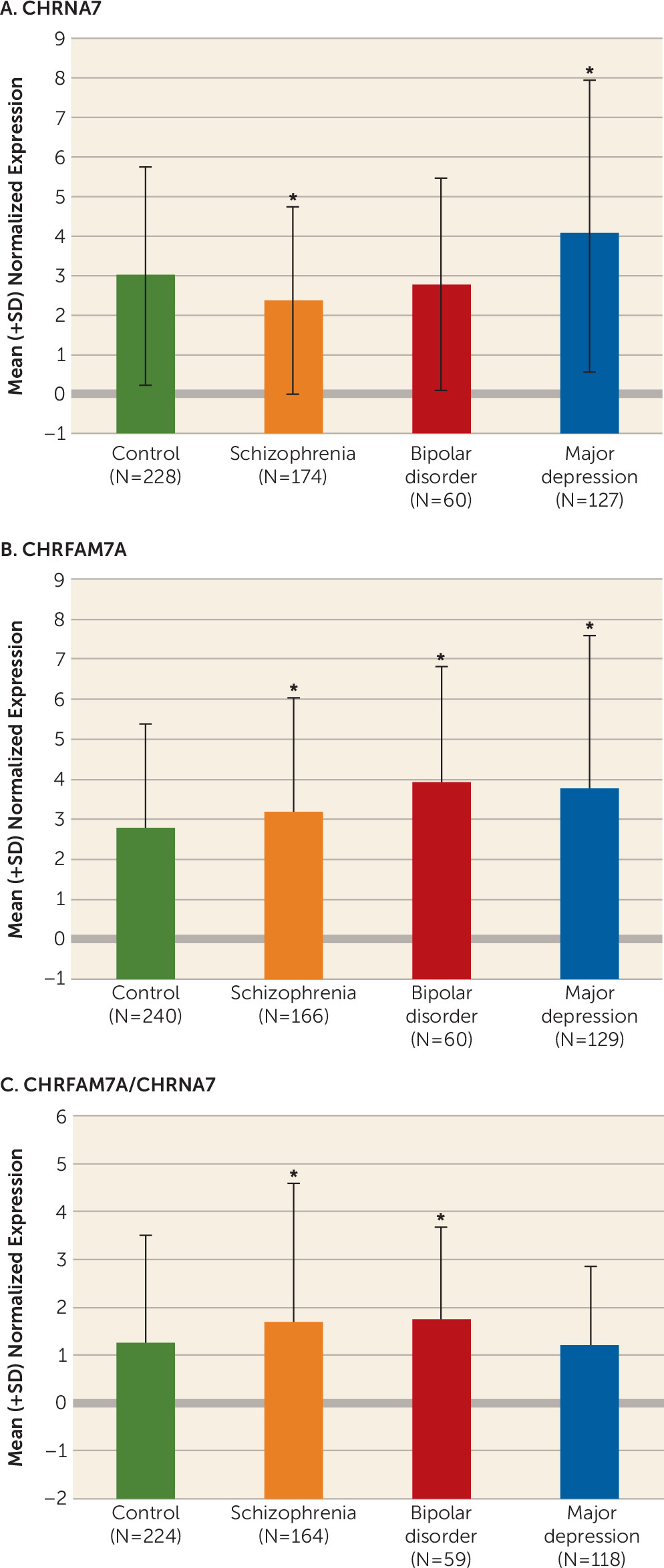
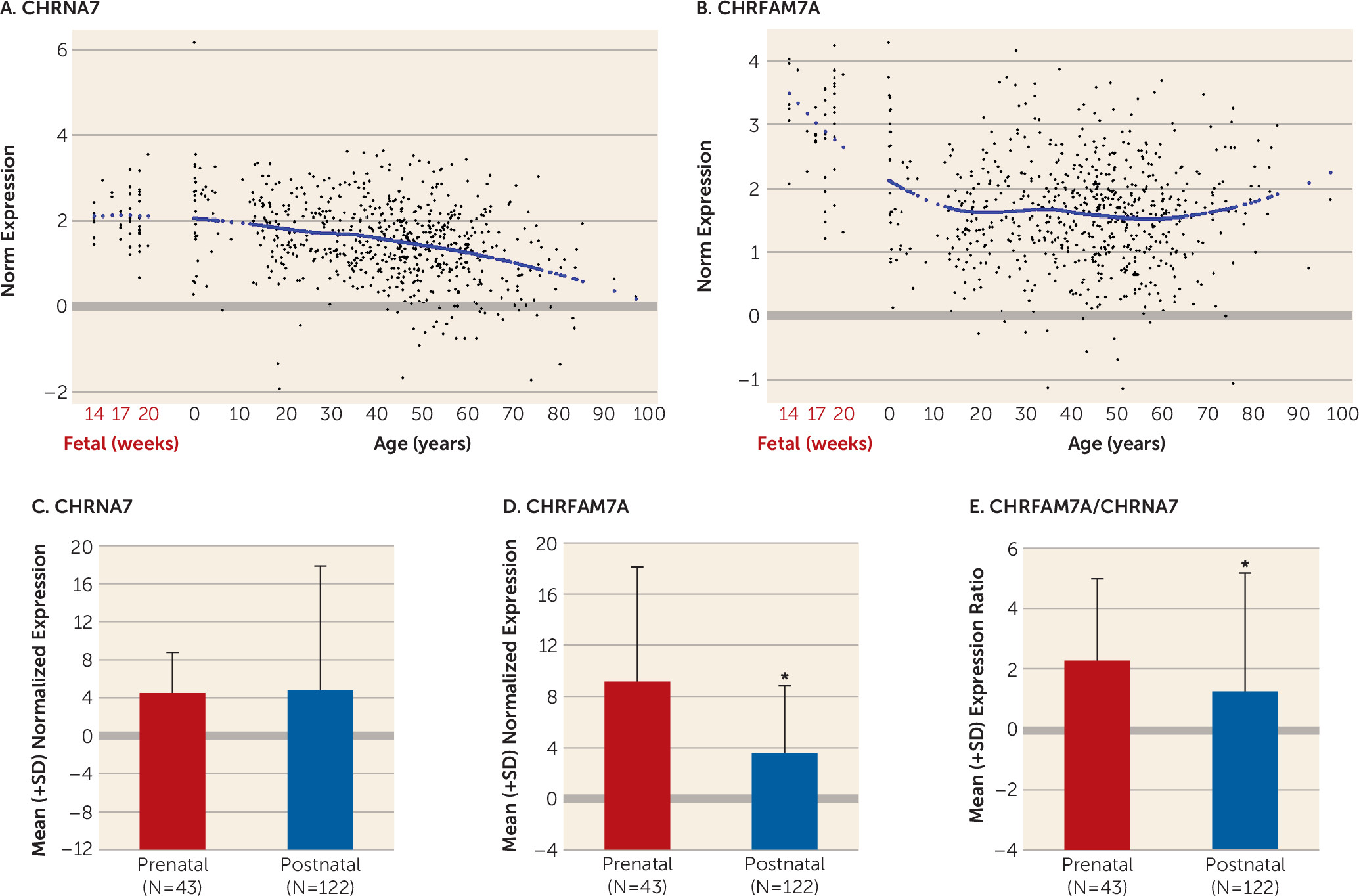
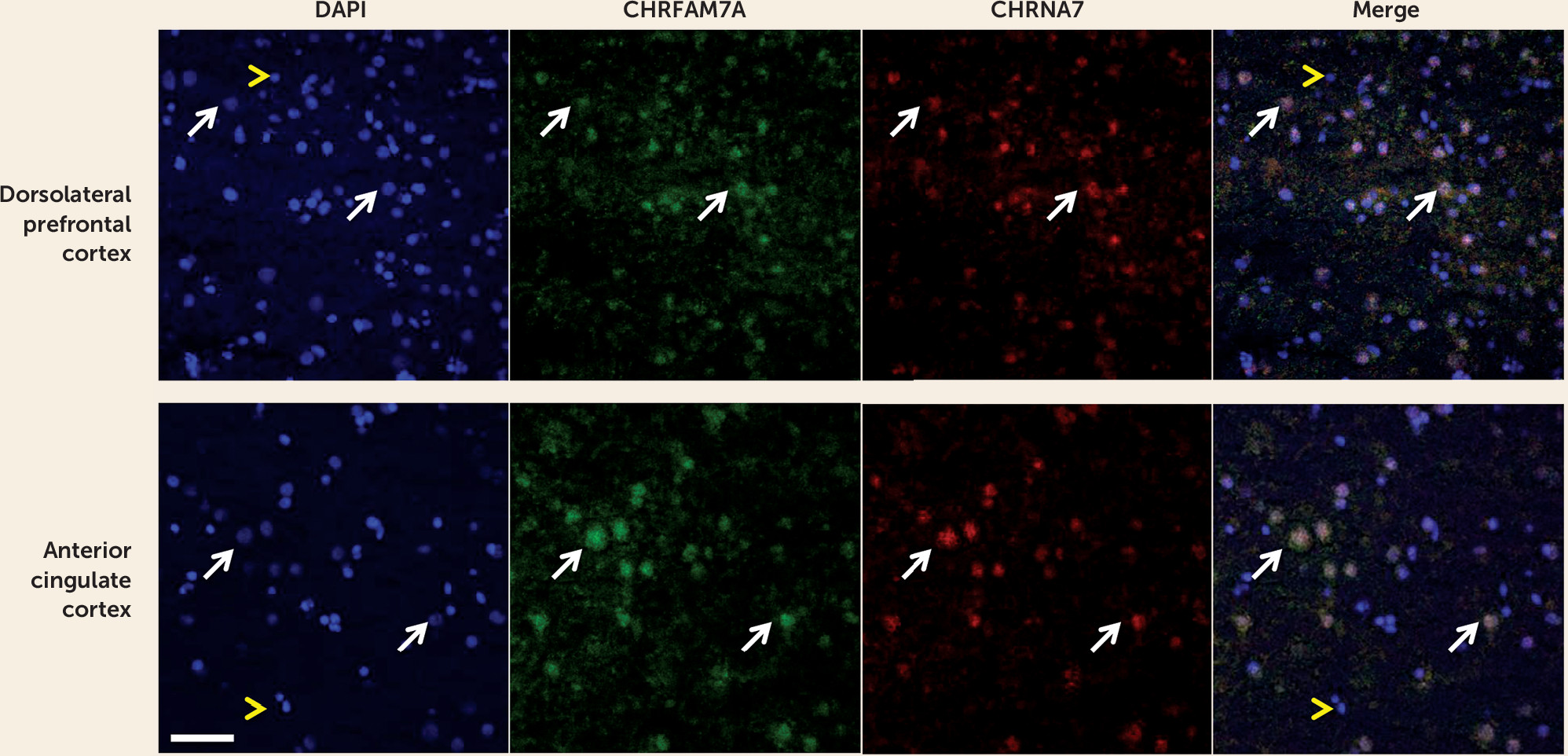
Our study is the first, to our knowledge, to show that CHRFAM7A is expressed in fetal human brain and that CHRFAM7A expression and CHRFAM7A/CHRNA7 expression ratios are markedly increased during the prenatal period compared with postnatal life. We are also the first to demonstrate coexpression of the two transcripts in a subset of neuronal cells in the human neocortex. Moreover, we found that CHRFAM7A/CHRNA7 expression ratios are significantly higher in the dorsolateral prefrontal cortex of patients with schizophrenia and bipolar disorder compared with normal comparison subjects due primarily to overexpression of CHRFAM7A in cases, a pattern of expression mimicking fetal life. Taken together, these findings suggest that the composition and function of α7 nACh receptors may be altered in the dorsolateral prefrontal cortex of patients with schizophrenia and bipolar disorder.
CHRFAM7A is considered to act as a dominant negative regulator of α7 nAChR function in humans (
11).
CHRFAM7A lacks
CHRNA7 exons 1–4, and in consequence,
CHRFAM7A protein is missing the signal peptide and part of the binding site for ligands. As a result, the receptor, which comprises the gene product of
CHRFAM7A, would not function as effectively as α7 nAChRs containing only the gene products of
CHRNA7. Indeed, in vitro oocyte-based studies have shown that the activity of α7 nAChRs is diminished (
32) and [
125I]-α-bungarotoxin binding is lower in cells coexpressing
CHRNA7 with the duplicate gene product (
11,
13). The mechanisms underlying these changes in receptor properties are not clear. It has been speculated that in addition to the formation of heteromeric receptors by these atypical duplicate subunits, they also interfere with oligomerization and assembly of α7 subunits in the endoplasmic reticulum, decreasing the number of mature α7 nAChRs able to migrate to the membrane (
13). It has been suggested that regulating the levels of cell surface α7 nAChRs by the human-specific duplicate subunit may be important for immune responses of the cholinergic anti-inflammatory pathway in the periphery (
33–
35). Furthermore, a recent report by Wang et al. (
36) provided evidence that the duplicated subunits are colocalized with full-length-7 subunits in the transfected mouse neuroblastoma cells (Neuro2a), as well as rat hippocampal neurons, are assembled and transported to the cell membrane together with full-length-7 subunits, and alter the function of the nAChRs. These findings support the view that the two gene products are colocalized and can function together. Our results of coexpression of the two transcripts in the human neocortex provide additional support for this concept.
The results of altered balance between
CHRNA7 and
CHRFAM7A in schizophrenia and bipolar disorder are in agreement with the only other published study on this topic, by De Luca et al. (
24), which showed nearly significant changes in the same direction in these disorders using the Stanley Foundation 35/35/35 postmortem dorsolateral prefrontal cortex collection (i.e., higher ratios of
CHRFAM7A/
CHRNA7 in case compared with comparison subjects). If indeed these altered
CHRFAM7A/
CHRNA7 ratios lead to functional changes, such as altered receptor assembly or receptor migration to the surface, they may perhaps explain reduced [
125I]- α-bungarotoxin binding reported in schizophrenia, as it would be expected that cells expressing the duplicated chimeric gene have a smaller number of binding sites (
11).
The results of our analysis of nicotine effects are not easy to interpret and reconcile with the existing literature. In contrast to our prediction based on the study by de Lucas-Cerrillo et al. (
13), who exposed cultured cells to nicotine in vitro and found significantly reduced expression of
CHRFAM7A, we did not detect changes in
CHRFAM7A expression in individuals who smoked or who were positive for nicotine at the time of death (
13). We also did not find an increase in
CHRNA7 expression in smokers with schizophrenia as reported previously in a relatively small postmortem study of the hippocampus (
37), but we used samples from a different brain region, and this may explain the discrepancy. On the other hand, we found contrasting changes between smokers and nonsmokers in two other diagnostic groups: an increase in
CHRNA7 expression in bipolar patients and a decrease in major depression patients who smoked compared with nonsmokers. These effects were weak, however, and did not survive corrections for multiple testing and thus may be spurious. In fetal subjects, we showed that
CHRNA7 expression tended to be decreased in subjects exposed to nicotine, but again these effects were weak and not statistically significant. Thus, although nicotine exposure during pregnancy has been associated with various obstetrical complications, such as spontaneous abortion, preterm birth, stillbirth, and low birth weight (
38) and with adverse effects on brain development, it is not clear whether any of these effects are mediated even partly through α7 nAChRs.
We found that the developmental trajectory of expression in the dorsolateral prefrontal cortex differed markedly between
CHRNA7 and
CHRFAM7A.
CHRFAM7A was relatively highly expressed during fetal life and then decreased dramatically from fetal age to young adulthood, whereas
CHRNA7 was more stable during these periods and decreased gradually with aging (
39–
41). This preferential fetal profile of expression of
CHRFAM7A suggests that
CHRFAM7A may play a yet unknown role in early brain development. Our findings of an altered balance between the fetal variant of the gene and the variant that constitutes a fully functional mature α7 nAChR are reminiscent of the recent findings suggesting delayed maturation of GABA signaling in patients with schizophrenia. In the hippocampal formation, GAD25/GAD67 and NKCC1/KCC2 ratios were increased in patients with schizophrenia compared with normal comparison subjects, reflecting a potentially immature GABA physiology (
42).
In this study, we did not find significant associations of
CHRNA7 and
CHRFAM7A expression with any of the SNPs examined, although previous genetic linkage analysis indicated that
CHRNA7 is associated with smoking in schizophrenia (
43) and several
CHRNA7 SNPs are associated with smoking in nonpsychiatric subjects (
44). D15S1360 polymorphism of the
CHRNA7 was also associated with susceptibility to nicotine dependence in subjects with schizophrenia (
45). We could not, however, detect a molecular basis for these clinical associations.
It is important to point out potential limitations of this study. As in any postmortem human brain examination, there are confounding factors, such as antemortem medication (antipsychotic drugs, antidepressants, lithium) or alcohol and illicit drug use (substance abuse was not an exclusion criterion for psychiatric patients, but it was for comparison subjects). Thus, it is possible that these factors contributed to or were responsible for the observed changes in patients. Another limitation is a lack of evidence in the human brain that CHRFAM7A mRNA is translated and that the protein product assembles in humans with α7 nAChRs.
In conclusion, we showed expression changes of CHRNA7 and CHRFAM7A and altered proportions of CHRFAM7A/ CHRNA7 in schizophrenia and bipolar disorder. We also demonstrated that the two transcripts are expressed together in a subset of cortical neurons. Given the evidence that the duplicated subunits interact with α7 nAChRs as well as other nAChRs (α3 and α4) and alter their function in the transfected cells, our results support the concept of aberrant function of nAChRs in mental illness.