Second, the specificity of reward network alterations with depressive symptoms is unclear. Depression has been conceptualized as an imbalance in dual valence systems (
5), involving reduced positive and/or increased negative affect. Etiologically, anhedonia is part of a positive valence system, whereas other depressive symptoms, such as low mood, are related to a negative valence system (
6). Based on existing evidence, reduced frontostriatal activations during reward anticipation should be specific to anhedonia—defined as diminished motivation or desire to engage in pleasurable activities (
7,
8). However, it is not clear whether reduced responses to reward in depression are exclusively associated with anhedonia or low mood, or if they are related to the combined effects of both symptoms. This has important implications, as anhedonia and low mood seem to be associated with differential treatment responses (
9,
10) and developmental trajectories (
11).
Here we address these outstanding questions by using functional MRI (fMRI) during a reward anticipation experiment (the monetary incentive delay task [
12]), in a large community-based sample of adolescents, using both a cross-sectional and a longitudinal design. This task is used to investigate reward anticipation and reward feedback (positive and negative outcomes). We focused on reward anticipation because the anticipatory phase, rather than the feedback phase, has been most strongly linked with anhedonia (
13), and we examined the feedback phase in exploratory analyses.
We first compared brain responses to reward anticipation between three carefully matched groups: adolescents with clinical depression, adolescents with subthreshold depression, and matched healthy volunteers. This allowed us to investigate how such brain responses vary across the spectrum of risk for depression. We hypothesized reduced activation in frontostriatal regions during reward anticipation in the clinical and subthreshold depression groups relative to the healthy comparison group, in keeping with findings from high-risk studies (
3,
14). We further hypothesized that brain activity in anticipation of reward differs in a graded manner across the risk spectrum, with participants suffering from clinical depression having the lowest neural activity. We also tested whether reduced activation in the reward network at baseline predicted clinical and subthreshold depression in previously healthy adolescents 2 years later.
We next investigated whether these reward network alterations are specific to anhedonia, as opposed to low mood, in a general-population sample. So far, no studies have examined this in adolescents, although some data exist from adult samples (
15). Previous studies suggested that depressed patients with anhedonia have lower dopaminergic activity in the caudate, putamen, and nucleus accumbens (
16) and that anhedonia is associated with exerting less effort toward attaining rewards (
7,
13). We hypothesized that alterations in the reward network are also present in adolescents with anhedonia, but not those who have low mood only. Finally, we examined whether reward network alterations differentially predicted development of anhedonia at the 2-year follow up.
Method
Participants
Neuroimaging and clinical data were obtained from the IMAGEN database, which covers eight sites in France, the United Kingdom, Ireland, and Germany and includes 2,223 adolescents recruited in schools around age 14. We used data from the first and second waves of IMAGEN. A detailed description of the recruitment and assessment procedures has been published elsewhere (
17). All local ethics research committees approved the study. Written consent was obtained from the parent or guardian, and verbal assent was obtained from the adolescent. After quality control for neuroimaging and behavioral tests, 1,576 adolescents were included in the study. Only a small minority of adolescents in this population-based sample were being treated with psychotropic medications (N=31, 2%).
Measures
Adolescent psychiatric symptoms and their impact were assessed with the Development and Well-Being Assessment (DAWBA), a self-administered diagnostic questionnaire consisting of open and closed questions (
18;
http://www.dawba.info). The DAWBA is designed to maintain consistency across multiple cultural and language groups, as diagnoses are made by clinical raters who share a common training and participate in regular cross-language training and consensus meetings. The DAWBA generates probabilities of having DSM-IV-TR diagnoses (
19), which we used to define the categorical diagnosis of depression.
The Strengths and Difficulties Questionnaire was used to assess general psychopathology and functional impairment (
20,
21).
All participants were coded as suffering from loss of interest/anhedonia or low mood if so rated (as “yes” or “no”) by self-report in the screening questions of the depression section of the DAWBA. These items have good face validity (see the data supplement that accompanies the online edition of this article).
Adolescents were included in a clinical depression group if they scored 4 or 5 on the DAWBA depression band (i.e., the highest probability of depression). In addition, following DSM-IV-TR criteria, these adolescents had to report at least five depressive symptoms, including at least one core symptom (abnormally depressed/irritable mood and/or loss of interest), and fulfill criteria for functional impairment and duration. Twenty-two adolescents met criteria for clinical depression at baseline.
Adolescents were included in a subthreshold depression group if in the past 4 weeks they had three or more depressive symptoms, including at least one core symptom and at least two other DSM-IV depressive symptoms, without fulfilling criteria for clinical depression in terms of duration, number of symptoms, or impact on functioning (
4). The same criteria were used to define depression groups at follow-up.
Participants with any current psychiatric diagnosis, with any history of depression or bipolar disorder, or with a score >5 on the Alcohol Use Disorders Identification Test were excluded.
At baseline, adolescents with clinical depression (N=22) and subthreshold depression (N=101) were compared with a group of healthy subjects (N=123) matched on age, sex, handedness, and imaging site. The healthy comparison group consisted of adolescents with fewer than three symptoms of depression and a probability of having a diagnosis of major depression of less than 0.1% according to the DAWBA (see Figure S1 in the online data supplement).
Other measures were collected using Psytools (Delosis, London), an online computer platform for self-assessment, including a pubertal status score using the computerized Pubertal Development Scale (
22).
Monetary Incentive Delay Task
To study neural responses to reward, we used a modified version of the well-established monetary incentive delay task (
12) that included three conditions: reward anticipation and receipt of positive or negative outcomes. A detailed description of the task is provided in the online
data supplement.
MRI Data Acquisition
Structural and fMRI data were acquired at eight IMAGEN assessment sites with 3-T scanners from various manufacturers (Siemens, Philips, General Electric, and Bruker). The scanning variables were specifically chosen to be compatible with all scanners. The same scanning protocol was used at all sites. In brief, high-resolution T1-weighted three-dimensional structural images were acquired for anatomical localization and coregistration with the functional time series. fMRI blood-oxygen-level-dependent (BOLD) images were acquired with a gradient-echo, echo-planar imaging sequence. For the monetary incentive delay task, 300 volumes were acquired for each subject. Each volume consisted of 40 slices aligned to the anterior commissure-posterior commissure line (2.4-mm slice thickness, 1-mm gap) acquired in a descending order. The echo time was optimized (echo time=30 ms, repetition time=2200 ms) to provide reliable imaging of subcortical areas. A detailed description of the fMRI data acquisition process is provided in the data supplement.
Statistical Analysis
We used coarsened exact matching in Stata, version 11 (StataCorp, College Station, Tex.), to create the matched healthy comparison group (N=123). After matching, no differences were found between the depressed groups (together, N=123) and the comparison subjects in sex, age, handedness, and imaging site.
To test our first hypothesis, the dimensionality of brain response to reward anticipation in adolescents as a risk factor for depression, we used one-way analysis of variance in SPM8 (Wellcome Trust Centre for Neuroimaging, London) to compare the clinical depression group (N=22) and the subthreshold depression group (N=101) with the matched healthy comparison group (N=123) at baseline, controlling for pubertal stage. Analyses were thresholded at an uncorrected p value of 0.001 at the voxel level, with clusters of activated voxels considered statistically significant at p<0.05, corrected for multiple comparisons. We used small-volume-corrected region-of-interest analyses across a fronto-striatal-limbic mask (see the online
data supplement). For all comparisons, brain locations were reported in terms of Montreal Neurological Institute coordinates, and the Wake Forest University PickAtlas (
23) was used to identify brain regions.
For subsequent analyses, we extracted the averaged beta values from the anatomical regions with significant clusters from the clinical- and subthreshold depression-matched group analyses using the MarsBaR toolbox (
http://marsbar.sourceforge.net) (
24). Whole regions from the Automated Anatomical Labeling atlas (
25) were used, with the exception of the ventral striatum, which we defined according to Martinez et al. (
26,
27).
We tested the dimensional hypothesis cross-sectionally, using a trend analysis (
28) of the gradual activation change in the significant clusters across unmatched healthy adolescents (N=1,453), adolescents with subthreshold depression (N=101), and adolescents with clinical depression (N=22).
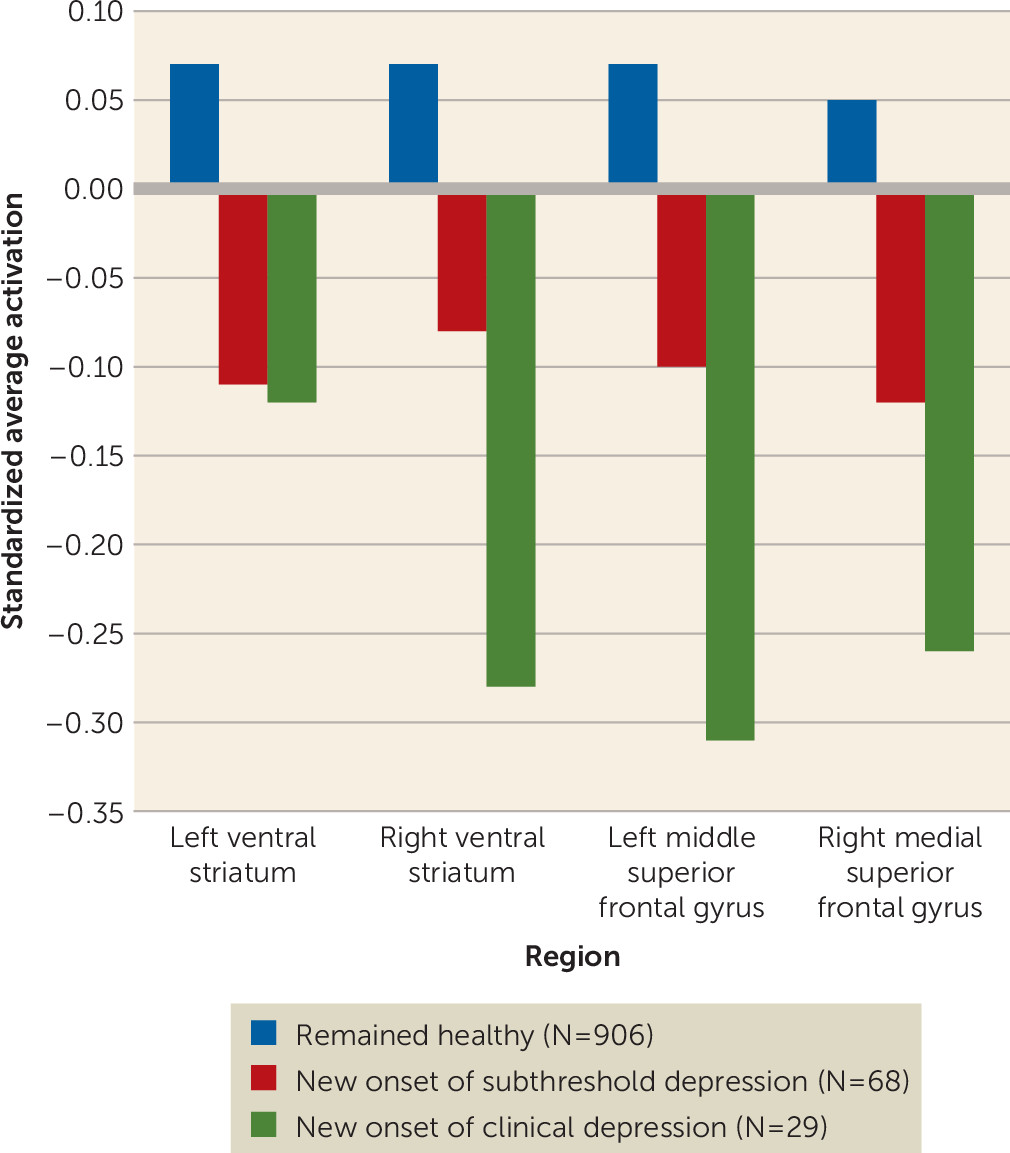
We predicted onset of subthreshold and clinical depression in previously healthy adolescents with logistic regression models with transition to subthreshold or clinical depression as the dependent variable and brain activations as the independent variable, adjusting for baseline depressive symptoms. We performed a trend analysis to examine the gradual change of activation in significant clusters in healthy adolescents across three groups: adolescents who remained healthy (N=906), those who developed subthreshold depression (N=68), and those who developed clinical depression (N=29) at the 2-year follow-up.
We used three methods to test the hypothesis that reduced ventral striatum activation is related to anhedonia but not to low mood. First, we compared brain activations between adolescents who reported anhedonia (N=254) and those who did not (N=1,322), using linear regression. Second, we compared brain activations between adolescents with (N=691) and without low mood (N=885). Third, we compared adolescents with only low mood (N=510), with only anhedonia (N=72), with low mood and anhedonia (N=183), and with no symptoms at all (N=536) using analysis of covariance. Because those with both symptoms—low mood and anhedonia—were more severely impaired (F=26.53, df=2, 762, p<0.001), the model was adjusted for functional impairment using the impact subscale of the Strengths and Difficulties Questionnaire.
To examine the specificity of response to reward with depressive symptoms, we used logistic regression models with type of symptom dimensions at 2-year follow-up as the outcome and brain activations as predictor of interest.
Analyses were completed in Stata, adjusting for sex, age, handedness, and pubertal status. The robust cluster option was used to adjust for the effects of imaging site and thus to account for the nonindependence of participants from the same site.
Results
Table 1 provides demographic and clinical information for the three groups at baseline and at 2-year follow-up.
Dimensionality of Brain Response to Reward Anticipation in Adolescents
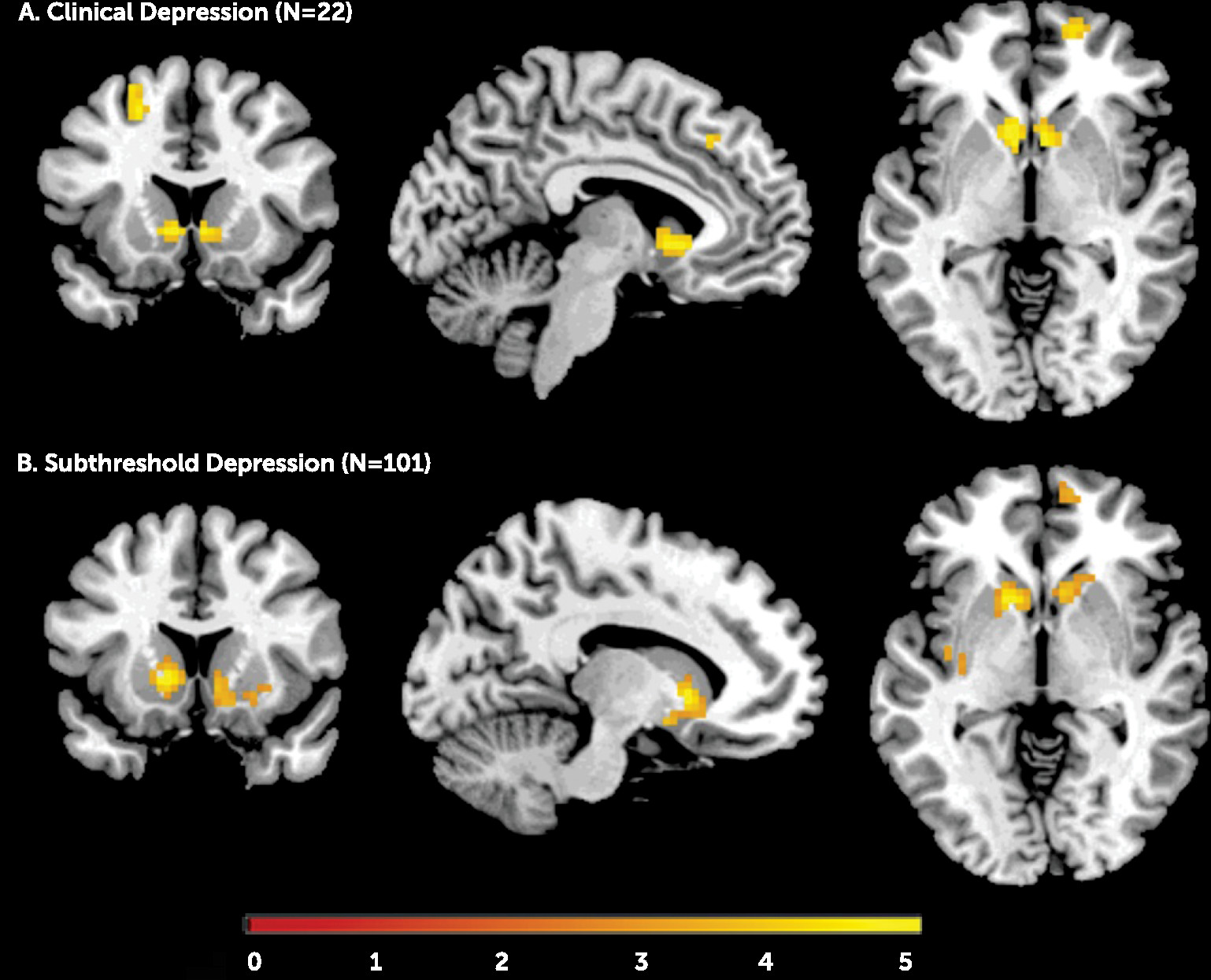
Adolescents with clinical depression showed less activation in the left and right ventral striatum, the right medial superior frontal gyrus, and the left middle superior frontal gyrus relative to the subjects without depression. Similarly, adolescents with subthreshold depression, relative to comparison subjects, had significantly lower activation in the ventral striatum bilaterally (
Figure 1; see also Table S1 in the online
data supplement). When we used a less conservative threshold (p<0.005 or p<0.01, both uncorrected), adolescents with subthreshold depression also showed reduced activation in the right medial and left superior frontal gyrus.
Based on these results, we extracted averaged beta values from the left and right ventral striatum, the right medial superior frontal gyrus, and the left middle superior frontal gyrus.
Sex, age, and pubertal status were not related to neural activations.
Trend analyses showed that brain responses to reward anticipation decreased gradually among the three groups (see Figure S3 in the data supplement) for the left (z=−3.02, p=0.003) and right ventral striatum (z=−2.89, p=0.004) and the right medial superior frontal gryus (z=−2.53, p=0.011).
Decreased activation in the ventral striatum predicted transition to subthreshold depression in previously healthy adolescents. Also, decreased activation in the right ventral striatum and the left middle superior frontal gyrus predicted transition to clinical depression 2 years later (
Table 2). Reduced ventral striatum response also predicted higher levels of depressive symptoms at follow-up according to the Adolescent Depression Rating Scale (see the
data supplement). All these results remained significant after accounting for depressive symptoms at baseline (
Table 2). Activations in the right medial superior frontal gyrus did not predict depression.
Trend analyses in healthy adolescents followed up over a 2-year period showed that activation in the right ventral striatum (z=−2.02, p=0.043), the left middle superior frontal gyrus (z=−2.38, p=0.017), and the right medial superior frontal gyrus (z=−2.02, p=0.044) decreased gradually across adolescents who remained healthy, those who developed subthreshold depression, and those who developed clinical depression (
Figure 2).
Specificity of Brain Response to Reward Anticipation in Adolescents in the Community-Based Sample
Adolescents with anhedonia (N=254) showed significantly decreased activation compared with those without anhedonia (N=1,322) in the ventral striatum bilaterally (left: β=−0.20, p<0.001; right: β=−0.16, p=0.015) but not in the left middle superior frontal and right medial superior frontal gyri. No significant differences in activation in the extracted regions were found between adolescents who reported low mood (N=691) and those who did not (N=885).
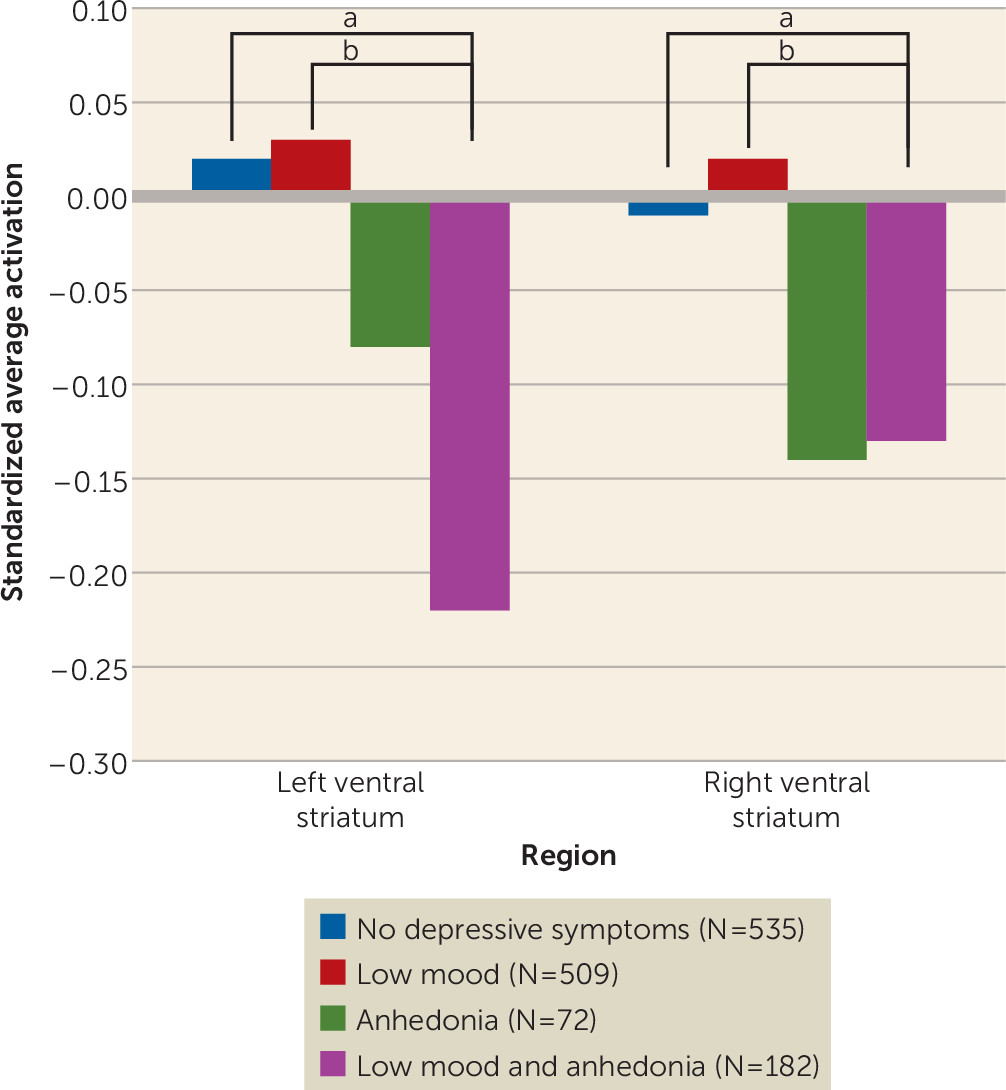
Next, we split the group of adolescents with anhedonia into those with low mood (N=182) and those without low mood (N=72). Adolescents with concurrent anhedonia and low mood symptoms had decreased activation in the ventral striatum bilaterally compared with adolescents with only low mood and adolescents without symptoms (
Figure 3). These results remained significant after adjustment for functional impairment. Full comparisons between groups in brain activations as well as in demographic and clinical variables are provided in Tables S2 and S3 in the online
data supplement.
In addition, there was a significant gradual decrease across healthy adolescents without anhedonia, healthy adolescents with anhedonia, adolescents with subthreshold depression, and adolescents with clinical depression in all examined regions except the left middle superior frontal gyrus (left ventral striatum: z=−3.51, p<0.001; right ventral striatum: z=−3.16, p=0.002; left middle superior frontal gyrus: β=−1.45, p=0.146; right medial superior frontal gyrus: β=−2.15, p=0.032) (see Figure S4 in the data supplement).
Based on the cross-sectional findings, we tested whether decreased reward network activations predicted anhedonia at follow-up. We found that decreased activation in the left ventral striatum predicted having concurrent anhedonia and low mood over low mood only (odds ratio=0.84, 95% CI=0.73, 0.98, p=0.030). Also, decreased activation in all four significant clusters predicted having both symptoms over no symptoms at all (left ventral striatum: odds ratio=0.79, 95% CI=0.65, 0.97, p=0.027; right ventral striatum: odds ratio=0.81, 95% CI=0.69, 0.94, p=0.007; left middle superior frontal gyrus: odds ratio=0.72, 95% CI=0.63, 0.82, p<0.001; right medial superior frontal gyrus: odds ratio=0.70, 95% CI=0.58, 0.85, p<0.001).
Adjusting the analyses for medication use had no effect on the results (data available on request).
Exploratory Analysis: Association of Depression and Anhedonia With Ventral Striatum Response to Positive and Negative Outcomes
Adolescents with subthreshold depression showed an increased response to positive outcomes on the fMRI task compared with matched healthy comparison subjects (left ventral striatum: β=0.30, p=0.037; right ventral striatum: β=0.36, p=0.019). No significant differences were found for clinical depression, anhedonia, or low mood (see Table S4 in the data supplement).
For negative outcomes, adolescents with subthreshold depression showed enhanced neural response (right ventral striatum: β=0.36, p=0.008). The same was true for adolescents with anhedonia (left ventral striatum: β=0.27, p<0.001; right ventral striatum: β=0.18, p=0.012). No significant differences were found for adolescents with clinical depression or low mood.
Discussion
In a large community-based sample of adolescents, we found that the reduced brain response to anticipation of reward in the ventral striatum varies across the risk spectrum for depression, is specific to anhedonia, and increases the risk for anhedonia and low mood as well as for depressive disorder at 2-year follow-up.
These findings of reduced activation during reward anticipation in frontostriatal regions, both in adolescents with subthreshold depression and in those with clinical depression, are consistent with findings from high-risk studies of depression (
3,
29). Our finding of a graded significant decrease in brain activation across groups suggests that frontostriatal response to reward anticipation operates as a mechanism across the spectrum of risk for depression.
Also, low ventral striatum activation was present only in those who had anhedonia, suggesting that this symptom is a behavioral marker of striatal activation. However, it should be noted that adolescents in the anhedonia-only group did not differ significantly in ventral striatal activation from those with low mood only or those without depressive symptoms. There were few participants with anhedonia only, and this may have underpowered our analysis.
The graded increase in the probability of depression with decreasing ventral striatum activation is akin to a dose-response relationship. Taken together with the specificity of the ventral striatum underactivation for anhedonia, it strengthens the inference that ventral striatum-based reward processing may be a mechanism—and therefore part of the causal chain—leading to depression. However, further steps—for example, the alleviation of depression through intervention targeting the ventral striatum—are required for establishing its role as a causal event, as opposed to a marker, in depressive illness.
We also explored the effects of positive and negative outcomes on the fMRI task. Consistent with previous studies (
30,
31), we found little evidence for alterations during positive outcomes. By contrast, we found that adolescents with anhedonia, but not those with low mood, showed increased activation in the ventral striatum during negative outcome. This is in keeping with previous results showing that anhedonia may be associated with sensitivity to punishment (
32). As these results were exploratory, they should be further replicated.
Depression is a heterogeneous syndrome with a variety of symptoms among which anhedonia and low mood appear as core features. According to the Research Domain Criteria framework, anhedonia is linked to the positive valence system, whereas low mood is linked to the negative valence system (
6). This division is not arbitrary; anhedonia and low mood are associated with different expression of symptoms (
33), developmental trajectories (
11), and clinical outcomes (
9,
10). Moreover, anhedonia has been associated with striatal functional connectivity involving the anterior cingulate cortex, whereas depression severity has been associated with striatal networks involving the ventromedial prefrontal cortex (
34). Interestingly, the medial prefrontal cortex, which also showed reduced response to reward anticipation in our depressed group (though it was not linked to anhedonia), is essential to integrating functions leading to “affective meaning” through its subcortical connections (
35,
36). Indeed, our findings on the ventral striatum and its specific link with anhedonia are consistent with the notion that regulation of positive affect is a critical feature of adolescent depression (
5).
Our results showed that a 1-point decrease in standardized ventral striatum activation increased the probability of future subthreshold depression by 20% and clinical depression by 35%, even when accounting for depressive symptoms at baseline. Moreover, a graded decrease was observed across healthy adolescents who, at 2-year follow-up, remained healthy, developed subthreshold depression, or developed clinical depression. In addition, decreased ventral striatum activation also predicted concurrent anhedonia and low mood at 2-year follow-up. Although the contribution to the variance of depression was small, our results suggest that reward circuit alterations precede the clinical expression of depression.
Although adolescents in the depression groups were more likely to be female and had a higher mean pubertal status score, it is unlikely that these differences could account for the neural findings. First, our main analyses were based on a sex-matched group. Second, all analyses in unmatched groups were adjusted for sex and pubertal status. Third, a post hoc analysis revealed that higher pubertal status score was associated with sex but not with depression, and there was no sex-by-depression group interaction. Finally, neither sex nor pubertal status score nor age was correlated with ventral striatum activations, although this may have been because 80% of our sample was between 14 and 15 years old and thus our analyses may have had insufficient variability.
This study should be seen in the light of several limitations. First, our fMRI task was based on monetary rewards rather than social rewards. Future longitudinal studies should distinguish between types of reward and depressive mechanisms. However, altered neural response to reward in depression is a remarkably consistent finding despite differences in fMRI paradigms and depression indices across studies (
37). Second, symptoms of anhedonia and low mood were each assessed with a single question, so we were unable to examine different degrees of severity. Third, the DAWBA only provides information about the past 4 weeks rather than lifetime depression. Fourth, because our follow-up data did not span the period of maximum risk for developing depression, we may have underestimated the extent to which neural activation may predict transition.