ECT is used to treat severe mental disorders in 1.4 million people annually worldwide, depression being the most common indication in Western countries (
1). ECT is the most acutely effective treatment for treatment-resistant, sometimes life-threatening, depression (
2,
3). Nevertheless, its use remains limited, mainly because of cognitive side effects (
4), especially concerns about retrograde amnesia (
5,
6).
Research on electrode placement has focused on preserving efficacy and minimizing side effects. Based on dosage, right unilateral ECT is less effective than bitemporal ECT (
2), the most commonly used electrode placement worldwide (
1), but causes less cognitive deficits (
7). High-dose is more effective than low-dose ECT but more adversely affects memory (
2,
7). However, efficacy trials (
8–
13) have demonstrated that unilateral ECT can be as effective as bitemporal ECT if delivered in high doses at multiples (e.g., 5×–8×) of seizure threshold, the minimum charge required to induce the generalized seizure needed for therapeutic effect.
Although unilateral ECT causes fewer cognitive side effects, the higher charges required to achieve comparable antidepressant efficacy might diminish its cognitive advantage. Relevant trials of brief-pulse (i.e., 1.0 msec–1.5 msec pulse width) ECT have obtained inconsistent results: some show comparable cognitive performance following high-dose (5×–8× threshold) unilateral ECT with reference to moderate-dose (1.0×–1.5× threshold) bitemporal ECT (
9,
10,
13), while others demonstrate less cognitive decline following high-dose (6× threshold) unilateral ECT (
8,
11,
12), although the latter studies mostly compared it with higher-dose (2.5× threshold) bitemporal ECT that increases cognitive side effects (
7). None of these studies were designed to determine unilateral noninferiority for antidepressant effect, and most had very limited follow-up. All used thrice-weekly treatment, common practice in the United States where most of these trials originated, even though this does not result in better outcomes than twice-weekly ECT (
14) but is associated with increased cognitive side effects (
15). This limits their generalizability for populations in which twice-weekly frequency is common practice, as occurs in many European countries (e.g., Belgium, Ireland, the Netherlands, United Kingdom) (
16,
17). Additionally, none of the previous trials reflected routine practice in that antidepressants were discontinued before ECT, and all but one (
9) required receiving at least eight ECT sessions unless response criteria were met.
To date, no randomized trial has tested whether twice-weekly high-dose (6× threshold) unilateral ECT is noninferior to reference (1.5× threshold) bitemporal ECT nor evaluated its superiority in terms of cognition and retrograde memory preservation over a prolonged follow-up period. We aimed to examine short- and long-term effectiveness and cognitive side effects of high-dose unilateral ECT compared with bitemporal ECT for severe depression in routine practice over 6 months.
Method
Study Design and Participants
EFFECT-Dep (Enhancing the Effectiveness of Electroconvulsive Therapy in Severe Depression) was a pragmatic, patient- and rater-blinded two-group parallel, randomized, noninferiority trial with a 6-month follow-up (
18,
19). Participants were all in-patients recruited between May 2008 and October 2012 from St. Patrick’s Mental Health Services (
http://www.stpatricks.ie/), an independent nonprofit organization that provides national services and runs Ireland’s largest ECT clinic, including referrals from public-sector hospitals. Eligible participants were ≥18 years old, referred for ECT, met diagnostic criteria for major depressive episode (unipolar or bipolar; Structured Clinical Interview for DSM-IV [
20]) and scored ≥21 on the 24-item Hamilton Depression Rating Scale (HAM-D) (
21). Exclusion criteria were conditions rendering patients unfit for general anesthesia or ECT; ECT in previous 6 months; history of schizophrenia, schizoaffective disorder, or neurodegenerative or other neurological disorder; alcohol/substance abuse in previous 6 months; involuntary status; and inability/refusal to consent. Treatment during the follow-up period was determined by patients in consultation with treating clinicians. This study was approved by the hospital’s research ethics committee (approval reference 012/07), and written informed consent was obtained after procedures were fully explained.
Randomization and Masking
After baseline assessments and before the first ECT session, patients were allocated (1:1) to bitemporal or unilateral ECT using an online system by the Clinical Trials Unit, King’s College London. Minimization with variable block sizes ensured that group allocation was balanced regarding three stratifiers: age >65 years (yes/no), previous ECT (yes/no), and referral site (St. Patrick’s Mental Health Services/St. James’s Hospital/other hospital). Recruiting researchers electronically submitted participants’ identifying number, initials, birthdate, history of ECT, and referral site. Treating clinicians received e-mail notification of randomization but were not involved in outcome assessments. Allocation was concealed from patients (prepared for receiving both electrode placements), recovery staff, referring clinicians, assessors, and trial statistician until completion of final analyses. Success of masking was investigated after end-of-treatment assessments by asking patients and raters to guess the treatment used.
Interventions
Brief-pulse (1.0-msec pulse width; current amplitude 800 mA) ECT was administered twice weekly (Mecta 5000M device, Mecta Corp., Portland, Ore.; maximum 1200 mC) according to Royal College of Psychiatrists’ guidelines, using methohexital (0.75 mg/kg–1.0 mg/kg) anesthesia, and succinylcholine (0.5 mg/kg–1.0 mg/kg) for muscle relaxation (
16,
22). Seizure threshold was established by dose titration at the first session (see the
data supplement accompanying the online version of this article). Subsequent treatments were 1.5×-threshold for bitemporal and 6×-threshold for unilateral (d’Elia placement) ECT. Stimulus charge was titrated upward as required during the treatment course (
22,
23). In line with regular practice, the number of ECT sessions was determined by referring clinicians in consultation with patients, up to 12 sessions in accordance with recommendations of the Irish Mental Health Commission (
http://www.mhcirl.ie/Mental_Health_Act_2001/Mental_Health_Commission_Codes_of_Practice/Use_of_ECT_for_Voluntary_Patients/), who publish annual national data on ECT (
http://www.mhcirl.ie/Publications/Publications/). Patients continued regular antidepressant treatments. ECT characteristics were recorded: seizure threshold (millicoulomb [mC]); mean stimulus charge (mC) for all sessions and nontitration sessions; motor and EEG seizure durations (seconds); total number of sessions; and number of sessions to establish threshold. Time to recovery of orientation (i.e., ability to answer 4/5 simple orientation questions, such as person, place, age, birthdate, day), once breathing spontaneously post-ECT, was recorded after each session (
24).
Common adverse physical effects (nausea, headache, muscle aches) were recorded for each session to measure occurrence (yes/no) within each course. Serious adverse events that required prolonged medical attention or were life-threatening were recorded.
Data were obtained at baseline, within, and soon after (2–4 days) completing the ECT course, as well as during the 6-month follow-up. Baseline assessments included the National Adult Reading Test (premorbid IQ), collection of demographic variables (age, gender, years in education, socioeconomic and marital status), and collection of the following clinical variables: referral reason, treatment resistance (yes/no; Antidepressant Treatment History Form [
25]), current psychotropic medications, number of previous depressive episodes, current episode duration, presence of psychosis, and depression polarity. Baseline Clinical Global Impression (CGI) severity was rated by referring clinicians.
Primary Outcome
The primary outcome was depression severity measured by the 24-item HAM-D after completing the ECT course (end of treatment). Interrater reliability for HAM-D scoring was assessed every 6 months; median intraclass correlation agreement was 0.96 (range: 0.89–0.98). To classify depression status, HAM-D scores were obtained after every second ECT session and 1 week after the final session if indicated.
Secondary Outcomes
Secondary depression outcomes included HAM-D scores at the 3- and 6-month follow-ups, end-of-treatment remission and response status, and relapse status for remitters during the 6-month follow-up. Remission was defined as ≥60% decrease from the baseline HAM-D score and a score ≤10 for two consecutive weeks; response was defined as ≥60% decrease from the baseline HAM-D score and a score ≤16; and relapse was defined as a HAM-D score ≥16 for two consecutive weeks. The majority of patients who relapse following successful ECT do so within 3 months (
26). To monitor relapse, HAM-D scores were obtained after the end of treatment at 2, 4, 6, and 8 weeks plus 3, 4, and 6 months.
The post-ECT cognitive secondary outcome of main interest, retrograde amnesia measured by the Columbia University Autobiographical Memory Interview-Short Form (
27), was prioritized for data collection. Further nonprioritized cognitive outcomes are summarized in Table S2 in the online
data supplement and included standardized measures of global cognition (Mini-Mental State Examination [MMSE]), auditory attention and verbal working memory (digit spans forward and backward), psychomotor speed and executive function (Trail-Making Test, parts A and B), semantic memory (category fluency), verbal learning and delayed recall (Free and Cued Selective Reminding Test), and visuo-spatial functioning and memory (Complex Figures Test). Alternative versions were used where appropriate. Cognitive outcomes were collected at baseline, end of treatment, and 3- and 6-month follow-ups. These outcomes, as well as the HAM-D scores, were similar to ones used to establish efficacy and side effects of bitemporal ECT (
7,
8,
11–
13,
16).
Subjective symptoms attributable to ECT were assessed with the Columbia ECT Subjective Side Effects Schedule, including six items on memory, concentration, and orientation for self-rating of cognition (total=18) (
28).
Sample Size
Based on a large bitemporal ECT series (
29), we estimated that 69 patients were required per group to have 80% power to demonstrate, using a one-sided equivalence t test at 5% level, that the mean reduction in the 24-item HAM-D score following high-dose unilateral ECT was no more than 4 points (i.e., equivalent to 3 points on the 17-item HAM-D, deemed to be clinically relevant [
30]) less than that achieved using bitemporal ECT.
Statistical Analyses
Analyses were on the intention-to-treat principle. ECT measures were summarized by trial arm using relevant descriptive statistics, accompanied by tests of zero group difference where this was not known a priori. We formally compared total numbers of sessions, numbers of sessions to establish seizure threshold (coded “1” or “≥2” sessions), and time to recovery of orientation using regression, logistic regression, and regression of log-transformed times, respectively. In these regression models, randomization stratifiers were included as explanatory variables in addition to trial arm.
The primary statistical analysis was assessment of difference in the 24-item HAM-D scores between arms at the end of treatment. The estimated group difference was supplemented by 95% confidence intervals and this interval compared with the noninferiority threshold (4 points). A regression model was fitted to end-of-treatment HAM-D measures, with prerandomization HAM-D scores, trial arm (unilateral/bitemporal), and randomization stratifiers as covariates. A similar analysis model was assumed for secondary HAM-D outcomes at the 3- and 6-month follow-ups. Among remitters, relapse during the 6-month follow-up was compared between arms using logistic regression as described above.
The main secondary cognitive outcomes of interest (Autobiographical Memory Interview at end of treatment and 3- and 6-month follow-ups) were analyzed using generalized linear models with a binomial distribution and logit-link (
31). Posttreatment Autobiographical Memory Interview measures provide the number of baseline items recalled after ECT (
27); such “number of items recalled” variables were therefore modeled as arising from binomial distributions, with maximum number of possible recalls set to the number of items obtained at baseline. An overdispersion parameter was introduced to account for recall of individual events being driven by subject characteristics. The covariates of these models were trial arm and randomization stratifiers.
Similar regression models were employed to describe nonprioritized continuous secondary outcomes: other cognitive tasks and subjective side effects (now also including baseline values as a covariate). Time outcomes (i.e., Trail-Making Tests, parts A and B) were log-transformed before analysis to acknowledge positively skewed distributions. The same approach was applied for count outcomes displaying positive skewness (subjective side effects total and cognitive scores). Group effects for these outcomes were therefore quantified by the ratio of outcome in the bitemporal arm to that in the unilateral arm.
For physical safety analyses, we assessed proportions of patients who had adverse events by treatment group and compared proportions using logistic regression modeling as for ECT measures.
Handling of missing data is described in the online data supplement. We used Stata (version 13) and SPSS 19.0 (IBM Corp., Armonk, N.Y.) for statistical analyses.
Results
Participant Flow
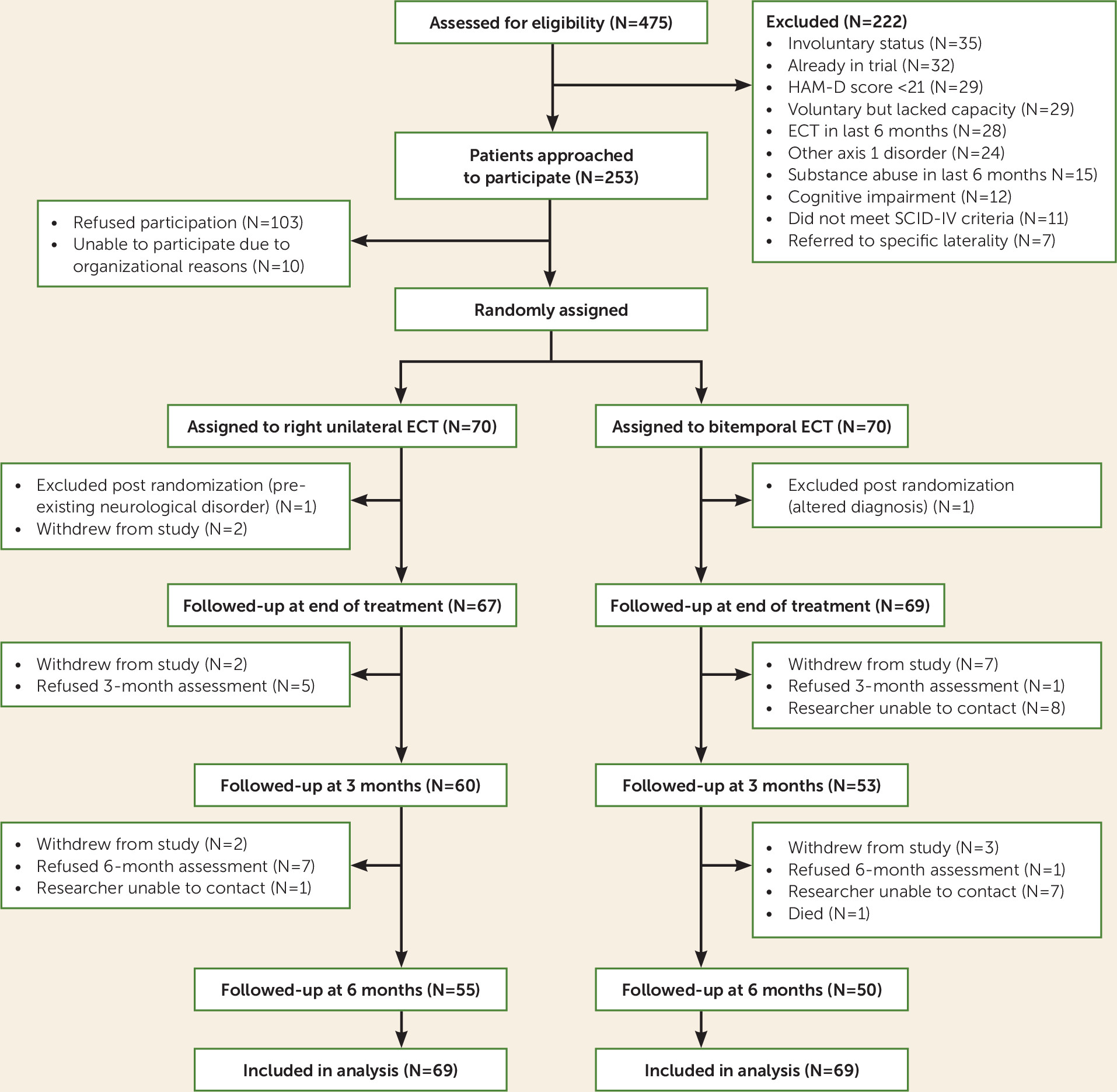
The trial profile is shown in
Figure 1. A total of 475 patients (mean age: 62 years [SD=15.1]; 67.7% female) were referred for ECT during the recruitment period (May 2008–October 2012), accounting for 32.9% of all ECT referrals in Ireland (N=1,480; average age: 57.3 years; 66.5% female). Seventy patients, all white Irish, were assigned per group. One patient per trial arm was excluded postrandomization because they were found not to fulfill eligibility criteria. Comparing the 138 participants to the 113 potentially eligible nonparticipants, participants were younger (mean age: 56.7 years [SD=14.8] versus 63.4 years [SD=14.3]; t=3.64, p=0.0001) but did not differ significantly regarding gender (% female: 63% versus 67%; χ
2=0.48, p=0.52), baseline CGI severity (mean: 5.3 [SD=0.7] versus 5.2 [SD=0.9]; N=101; U test: z=0.93, p=0.35), and MMSE scores (mean: 27.7 [SD=2.1]; N=119 versus 27.8 [SD=2.5]; N=85; t=−0.27, p=0.79).
All patients adhered to allocated treatment, although five (7.2%) unilateral patients had thresholds >200 mC (225 mC, N=1; 250 mC, N=3; 500 mC, N=1) and thus could not be treated at a fully 6× seizure threshold. Nearly all participants (N=136; 98.6%) were assessed for primary outcome at the end of treatment, while 82% and 76%, respectively, were followed up at 3 and 6 months.
Treatment guesses were made by patients (119/138) and raters (118/138): 12 patients could not guess, and 26/56 in the unilateral group and 36/51 in the bitemporal group correctly guessed (χ2=3.27, p=0.07; kappa=0.17 [low coefficient of beyond-chance agreement]). For raters, 30/57 of the guesses for the unilateral group and 36/61 for the bitemporal group were correct (χ2=1.61, p=0.21; kappa=0.12). Thus, masking was successful for patients and raters.
Baseline and Treatment Characteristics
Summaries of baseline characteristics were comparable between trial arms as would be expected under random allocation (
Table 1). Age (mean: 56.7 years [SD=14.8]), gender (63% female), psychosis status (21%), bipolarity (23%), baseline 24-item HAM-D scores (mean: 29.9 [6.2]), and depression episode median duration (19.5 weeks) for the total sample were similar to that found in previous relevant trials (
8,
9,
11–
13) and large observational studies (
5,
29).
Anesthesia doses were similar for the two groups (
Table 2). In line with previous studies (
8–
12), we found that threshold was lower with unilateral ECT, and total stimulus charges were higher in the unilateral group, while seizure durations were similar between groups. Ninety-three percent of patients had an adequate seizure in the first session. Although it took fewer sessions to establish threshold in the unilateral group (p=0.002), there was no significant difference between groups for total number of ECT sessions (p=0.26). Median time to recovery of orientation following the initial titration session in the unilateral group was half that of the bitemporal group (p<0.001), and this cognitive advantage was maintained, though to a lesser extent, during the remainder of the course.
Primary and Secondary Mood Outcomes
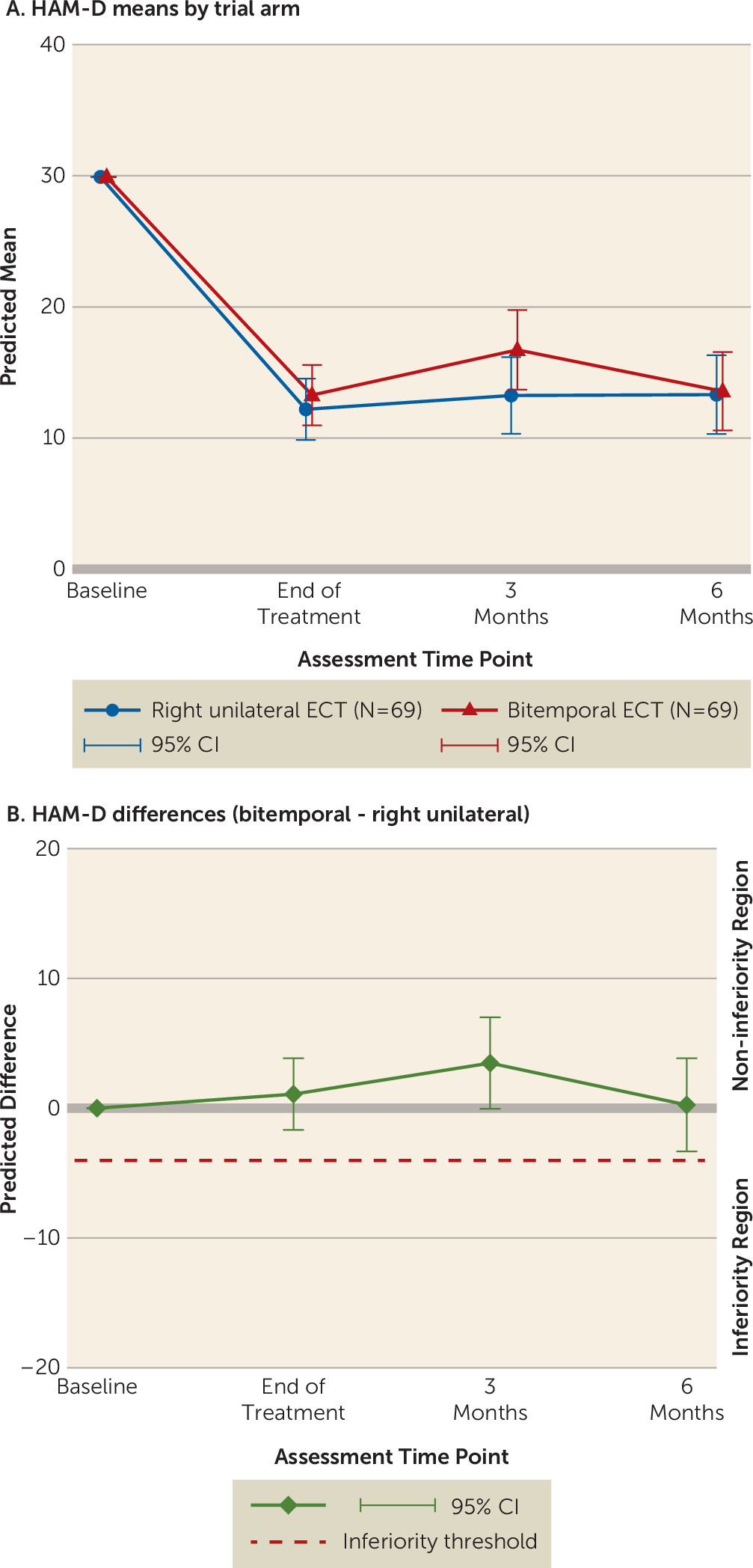
High-dose unilateral ECT was noninferior to bitemporal ECT at the end of treatment. Changes in the 24-item HAM-D scores are shown in
Figure 2A. The prespecified noninferiority margin was no more than a –4-point difference at the end of treatment between the bitemporal and unilateral groups (
Figure 2B). The predicted difference at the end of treatment was 1.08 (95% confidence interval [CI]=–1.67 to 3.84; unilateral, N=67; bitemporal, N=69). Noninferiority was evident at both the 3-month (3.48, 95% CI=–0.046 to 7.0; unilateral, N=60; bitemporal, N=53) and 6-month (0.26, 95% CI=–3.33 to 3.85; unilateral N=55, bitemporal N=50) follow-ups.
These results translated into similar proportions of responders (unilateral: 42/69 [60.8%]; bitemporal: 35/69 [50.7%]) and remitters (unilateral: 32/69 [46.4%]; bitemporal: 29/69 [42.0%]) in the two groups at the end of treatment. The median number of ECT sessions for both responders and remitters was 7 (range: 3–12), which was less than the median number of 9 (range: 3–12) ECT sessions for both nonresponders and nonremitters (for both Mann-Whitney U test, p<0.001). During the 6-month follow-up, there was no significant difference between the proportion of remitters who relapsed in the unilateral (8/32; 25.0%) and bitemporal (11/29; 37.9%) groups (odds ratio [unilateral/bitemporal]=0.56, 95% CI=0.17–1.79, z=0.99, p=0.32).
Cognitive Secondary Outcomes
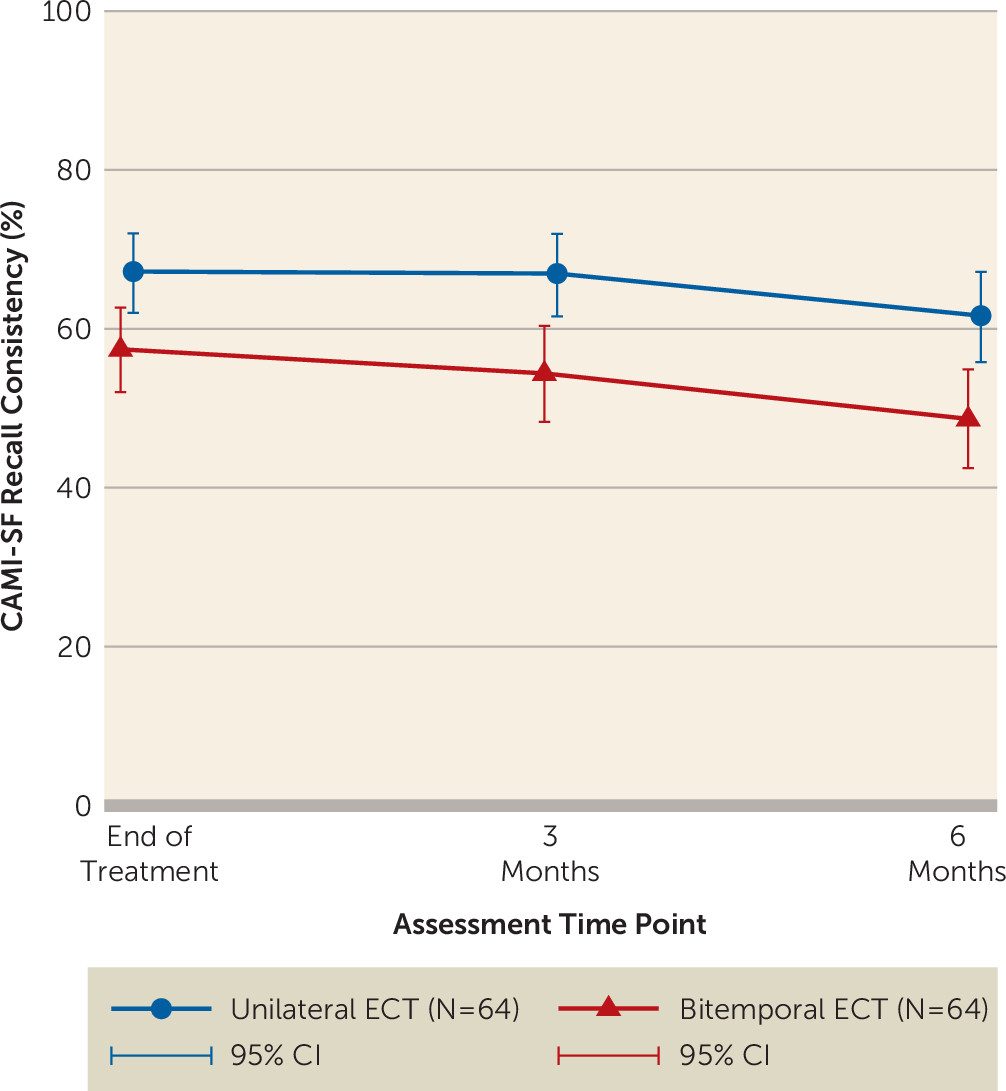
The cognitive outcome of main interest post-ECT was retrograde amnesia as measured by the Autobiographical Memory Interview. Autobiographical memory scores for the unilateral (46.9 [SD=9.7]; N=66) and bitemporal (44.4 [SD=10.3]; N=64) groups were similar at baseline. The percent consistency of recall of baseline memories was lower in the bitemporal group at the end of treatment (odds ratio=0.66, 95% CI=0.513–0.85, p=0.001; unilateral, N=64; bitemporal, N=64), and this was maintained at follow-up after 3 months (odds ratio=0.59, 95% CI=0.45–0.78, p<0.001; unilateral, N=56; bitemporal, N=48) and 6 months (odds ratio=0.59, 95% CI=0.45–0.79, p<0.001; unilateral, N=49; bitemporal, N=42) (
Figure 3). Distributions of individual percent recall consistencies are presented in Table S3 in the online
data supplement.
Assessment completion levels varied for nonprioritized secondary outcomes. End-of-treatment completion rates ranged from 93.5% (category fluency) to 71.7% (verbal learning). Three-month completion rates varied from 62.3% (category fluency) to 47.8% (Trail-Making Test, part B) and at 6 months from 59.4% (category fluency) to 42.8% (Trail-Making Test, part B).
There were few differences between groups for the other cognitive tasks (see Table S4 in the data supplement). At the end of treatment, the only statistically significant difference was for better performance in the unilateral group on verbal learning for immediate recall (p=0.034), though not delayed recall (p=0.22). There were no differences between groups on these verbal learning and memory tasks at the 3- and 6-month follow-ups. At 3 months, performance was better in the unilateral group for both auditory attention (p=0.021) and verbal working memory (p=0.049), but these cognitive advantages were not evident at the end of treatment or at the 6-month follow-up.
There were no significant differences between groups on the Subjective Side-Effects Schedule for total side effects at any time point (see Table S4 in the
data supplement), although the number and severity of side effects declined substantially over time, probably in line with improved mood (
16,
28). However, significantly fewer subjective cognitive side effects were reported by the unilateral group at the end of treatment (p=0.02) and after 6 months (p=0.025). Thus, there were both objective and subjective cognitive advantages for unilateral compared with bitemporal ECT.
Adverse Events
There were no differences between the unilateral and bitemporal groups for occurrence of headaches (26.5% versus 27.5%; odds ratio=0.93, 95% CI=0.42–2.04; z=0.20, p=0.84), nausea (16.2% versus 11.6%; odds ratio=1.54, 95% CI=0.56–4.17; z=−0.84, p=0.40), or muscle pain (11.8% versus 8.7%; odds ratio=1.37, 95% CI=0.44–4.17; z=−0.55, p=0.58).
Regarding major adverse events, six patients required β-blockers for ECT-related hypertension (unilateral, N=4; bitemporal, N=2). In the unilateral group, one patient developed laryngospasm with temporary drop in oxygen saturation, one developed tachyarrhythmia necessitating ECT termination, and one attempted suicide during the course. In the bitemporal group, three patients developed interictal confusion resulting in postponement/termination of ECT, one developed bronchospasm, one required β-blocker treatment for sinus tachycardia, one developed bradyarrhythmia, and one developed a pulmonary embolus after the fifth treatment. None of these events led to trial dropout.
Discussion
Our findings show that twice-weekly high-dose unilateral ECT is noninferior to bitemporal ECT for severe depression in regular clinical practice, which included continued antidepressant pharmacotherapy, and this was maintained over 6 months. The proportions of responders and remitters, as well as relapse rates, are consistent with this. Furthermore, we found high-dose unilateral ECT to be less taxing on autobiographical memory than bitemporal ECT. The unilateral group showed significantly higher autobiographical memory consistency with baseline recall than the bitemporal group at the end of treatment and the 3- and 6-month follow-ups. Other cognitive advantages of unilateral ECT included quicker recovery of orientation following treatments, better verbal learning at the end of treatment, and fewer subjective cognitive side effects. Both forms of ECT were well tolerated. The numbers of common physical side effects and serious adverse events were similar in both groups, in line with previous studies reporting harms (
12,
32).
Our findings for the primary outcome, the 24-item HAM-D score, are consistent with results of previous, nonpragmatic, thrice-weekly efficacy trials (
8–
13). However, the overall remission rate (44.2%) was lower than the rate in some trials (range: 46%−65%) (
8,
9,
11–
13) but similar to that in a large community study (46.7%) (
33), while the overall 6-month relapse rate (31.1%) was at the lower limit reported in a recent meta-analysis of post-ECT relapse (
26). These differences most likely reflect the pragmatic nature of our trial, in which the number of treatments was decided by the patients and referring physicians rather than by the protocol, as well as a naturalistic follow-up, and are unlikely to be related to concomitant use of antidepressants, which may improve ECT efficacy (
12). Cognitive outcomes at the end of treatment were consistent with previous thrice-weekly ECT trials (
8,
9,
11,
12). Regarding autobiographical memory, as measured by the Autobiographical Memory Interview, our findings differed only from two previous trials (
9,
13) that found both treatments to have comparable effects. This might be explained by the higher stimulus charge used for the unilateral group (8× seizure threshold) for one trial (
9) and/or use of thrice-weekly ECT (
9,
13), as both result in larger cognitive deficits.
Strengths and Weaknesses
Trial strengths include noninferiority design, pragmatic attitude, relatively large sample size, and adequate power. We showed excellent adherence and end-of-treatment completion rates. Retention at both follow-ups was satisfactory for the primary and main cognitive outcomes and superior to previous relevant trials. Indeed, existing efficacy trials either lacked follow-up (
8,
12,
13), had shorter follow-ups (1–2 months) (
9,
10), and/or had smaller follow-up samples (19–22 per group) (
9,
11). None was designed to test noninferiority of high-dose unilateral ECT compared with bitemporal ECT. The randomized sample was representative of the general population referred for ECT and similar to potentially eligible nonparticipants. Our findings, therefore, have good generalizability to countries where twice-weekly ECT is normal practice.
Our study has some limitations. First, we did not include involuntary patients who could not consent due to illness severity (7.4% of referrals), for whom bitemporal ECT may be better (
13). Second, other than for autobiographical memory, there are high levels (13%−54%) of missing variables for secondary cognitive outcomes at the follow-ups. Nevertheless, this study presents the best available evidence, to our knowledge, of long-term cognitive correlates of high-dose unilateral and bitemporal ECT. A third limitation concerns the Autobiographical Memory Interview. We selected this instrument to situate our trial within existing research evidence, as most previous trials used a variant of it (
8,
11–
13). However, it does not allow quantification of retrograde amnesia attributable directly to ECT even though it is sensitive in detecting differences between treatment allocations on autobiographical memory recall (
6,
7,
34,
35). Nevertheless, the present trial shows that high-dose unilateral ECT affects autobiographical memory less than bitemporal ECT. Fourth, all trial participants were in-patients, but this reflected routine practice. Fifth, the relatively lower remission rate may be due to the pragmatic design when compared with other trials (
8–
13) performed under more stringent, but less clinically general, conditions.
Conclusions
Our study has important clinical implications. In terms of harms/benefits ratio, high-dose unilateral ECT was noninferior to bitemporal ECT but showed a better cognitive profile, especially for preserving retrograde personal memories and fewer subjective cognitive side effects. While there is much interest in other modifications to maintain effectiveness but reduce side effects (e.g., ultrabrief pulse-width ECT), these require further refinement and characterization for optimization (
36,
37). Our findings justify considering high-dose unilateral ECT as the preferred ECT option for treating depression and may help improve acceptability and availability of this effective treatment.