Recent investigations using animal models suggest that the development of psychosis is associated with increased resting activity in the hippocampal region, and that this is linked to an increase in efferent activity in local glutamatergic neurons that project to the basal ganglia and midbrain (
1), elevating activity in areas responsible for dopamine signaling (
2,
3). Animal models would thus predict that, prior to the onset of psychosis in humans, resting activity may be elevated in the hippocampus, midbrain, and striatum (
4). Neuroimaging studies in people at high risk of developing psychosis have yet to explicitly test this hypothesis (
4). However, in subjects at high risk for psychosis, the volume of the hippocampal region is reduced (
5,
6), local resting blood volume is increased (
7), and the hippocampal response to a range of cognitive tasks is altered (
8,
9). Furthermore, high-risk subjects also show elevated dopamine function in the midbrain and the striatum (
8,
10), as well as a perturbation in the normal relationship between activation in the hippocampus and striatal dopamine function (
11).
The first aim of the present study was to test the hypothesis that subjects at ultra-high risk for psychosis would show increased resting state regional cerebral blood flow (rCBF) in the hippocampus, basal ganglia, and midbrain. We measured rCBF, which provides an indirect measure of neuronal function (
12), using pseudo-continuous arterial spin labeling (pCASL). Measurements of rCBF show the intimate relationship between neuronal activity and the control of blood supply, a process known as neurovascular coupling (
13), and provide an indirect but highly correlated measure of neuronal function (
12).
Our second aim was to examine the relationship between rCBF in these regions and longitudinal changes in the severity of psychotic symptoms. There is marked heterogeneity in the clinical course of ultra-high-risk subjects after presentation (
14,
15), with a substantial proportion improving to the extent that they no longer meet criteria for an ultra-high-risk state. In other cases the presenting symptoms persist or progress to the extent that there is a first episode of frank psychosis (
15). Our second hypothesis was that changes in the severity of psychotic symptoms following presentation would be related to longitudinal changes in the level of resting perfusion in the hippocampus, basal ganglia, and midbrain.
Discussion
Our first hypothesis was confirmed; in subjects at ultra-high risk for psychosis, rCBF within the hippocampus, basal ganglia, and midbrain was significantly greater than in healthy comparison subjects. Although there were also group differences in global gray matter rCBF, these were not significant after controlling for group differences in anxiety levels and tobacco use (
27,
28), while group differences in regional perfusion remained significant after global rCBF and other potentially confounding variables were included as covariates.
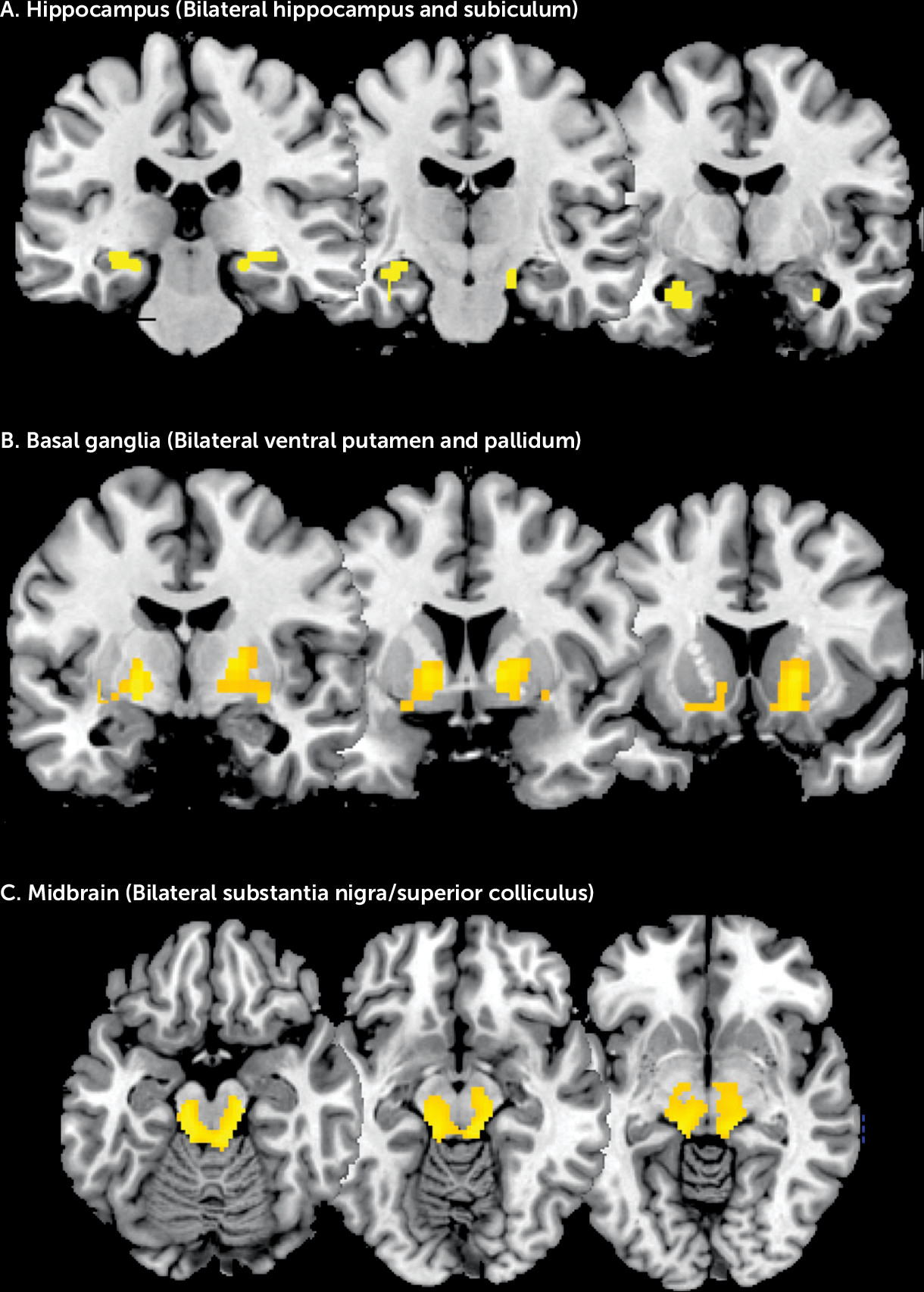
Our regional predictions were based on rodent models of psychosis that propose that increased excitatory activity in the hippocampus drives increased subcortical dopamine function through effects on the midbrain and striatum (
29). While there is extensive evidence from experimental studies in animals to support this model (
4,
29–
31), relatively few neuroimaging studies have sought to explicitly examine it in humans (
4). In the present study, we observed elevated rCBF in the hippocampus and subiculum extending to the anterior hippocampus, the ventral putamen and pallidum, and the part of the midbrain that includes the substantia nigra. This regional distribution of increased perfusion corresponds to the network of areas implicated in animal models of psychosis (
3,
8,
32). Regions within this putative network have also been identified in previous neuroimaging studies of ultra-high-risk subjects as sites of reduced gray matter volume (
6), increased resting blood volume (
33), and altered activation during cognitive tasks (
8,
9), while dopamine function has been found to be elevated in the midbrain and striatum (
34).
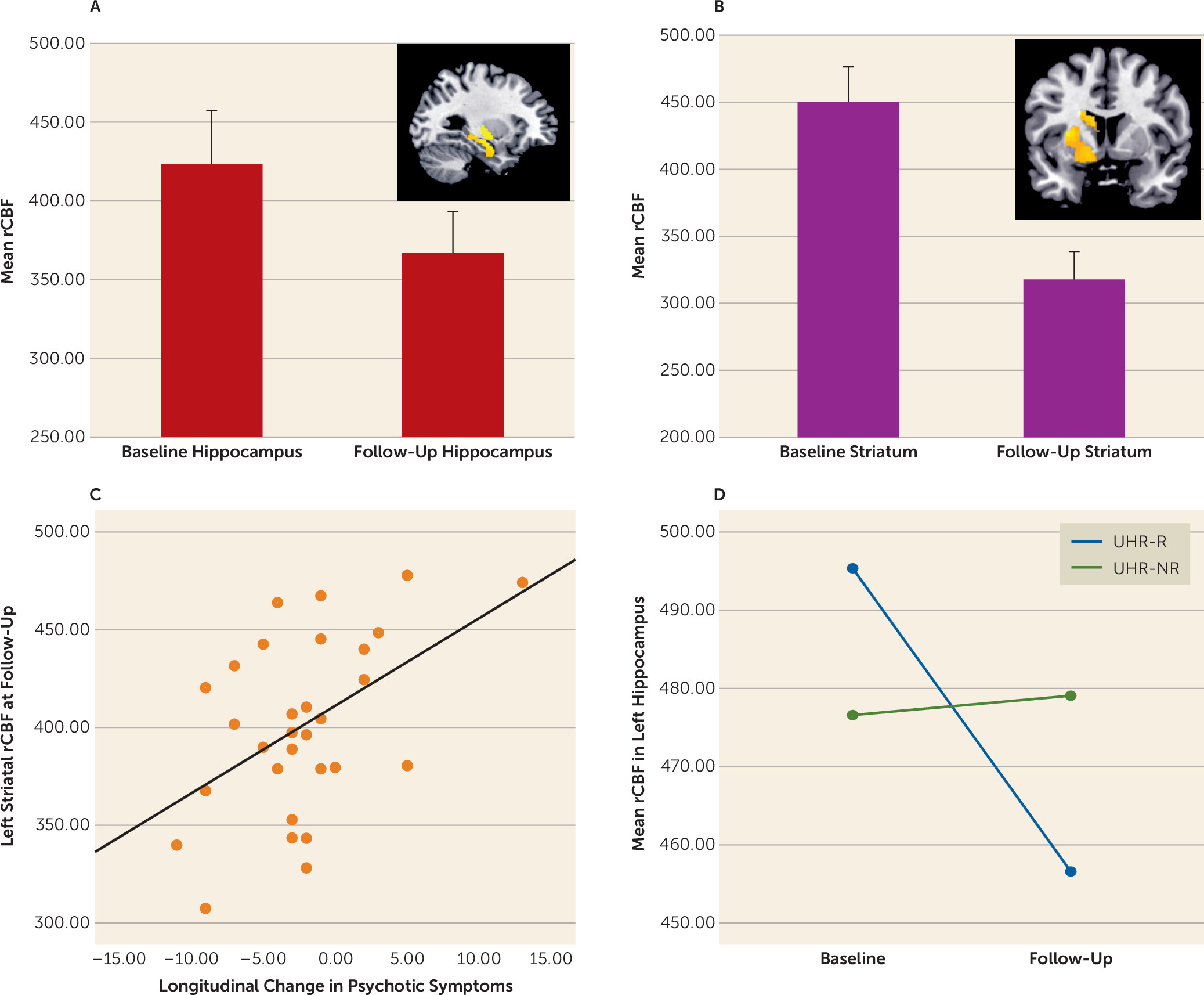
Our second prediction was that changes in the severity of psychotic symptoms in ultra-high-risk subjects following clinical presentation would be related to longitudinal changes in rCBF in the same subjects. The ultra-high-risk sample as a whole showed a symptomatic improvement, along with a concurrent longitudinal reduction in rCBF in the hippocampus and caudate bilaterally and in the left putamen. This is consistent with evidence that increased activity in subcortical areas involved in dopaminergic signaling underlies the generation of psychotic symptoms (
29). More interestingly, although the overall severity of symptoms in the ultra-high-risk group improved, the pattern of symptomatic change within the sample was markedly heterogeneous; in about half of those followed up, there was a reduction in symptoms such that they no longer met criteria for an ultra-high-risk state (i.e., they were in remission). In contrast, the symptoms in the other half of this group either persisted or worsened, in some cases to the point where they became frankly psychotic. Comparison of these two subgroups revealed that there was a greater longitudinal reduction in hippocampal rCBF in those who entered remission than in those who did not. This finding is in line with previous neuroimaging data linking alterations in medial temporal structure and function with an adverse clinical outcome in high-risk subjects (
5,
6,
8).
Previous ASL and positron emission tomography studies of resting perfusion in schizophrenia have also described elevated rCBF in striatal (
35–
37), midbrain, and hippocampal (
38) regions, although this has often been accompanied by cortical reductions in rCBF, particularly in the prefrontal cortex. In the present study, a supplementary analysis (see the online
data supplement) revealed increased, rather than decreased, prefrontal cortex rCBF in ultra-high-risk subjects relative to comparison subjects. Indeed, we did not find reductions in rCBF in any region in our ultra-high-risk sample. This contrast with findings of reduced resting prefrontal cortex perfusion in established schizophrenia raises the possibility that reduced prefrontal cortex rCBF may become evident after the onset of the illness, perhaps as an effect of treatment and/or as pathological mechanisms associated with chronicity.
Although our longitudinal analyses focused on comparisons between subgroups of ultra-high-risk subjects who did and did not show symptomatic improvement, there was also a significant reduction in attenuated positive, negative, and total symptoms in the ultra-high-risk sample overall. This suggests that the normalization of rCBF following presentation may be related to a longitudinal reduction in symptom severity. The basis of widespread cortical and subcortical hyperperfusion at baseline is beyond the scope of the present study, but previous work in both experimental animals and ultra-high-risk subjects suggests that this may reflect perturbations in cortical GABA and glutamate function (
1,
4,
33,
39). Although speculative, it is possible that global hyperperfusion is seen in ultra-high-risk cases but that the hypoperfusion abnormalities seen in established schizophrenia are the consequence of excitotoxic effects due to continual cortical disinhibition (
33). This prediction could be examined in longitudinal multimodal neuroimaging studies in ultra-high-risk cohorts.
Potential Limitations
Most of our ultra-high-risk subjects were medication-naive, but a minority (seven of 52) had been treated with low dosages of antipsychotic drugs, which could have altered the severity of psychotic symptoms and rCBF (
24). However, the number of medicated subjects was small, and they were equally distributed between the two ultra-high-risk subgroups with different clinical outcomes. Moreover, supplementary analyses showed that the findings remained significant after exclusion of the ultra-high-risk subjects who had received antipsychotic medication. The proportion of the ultra-high-risk sample who developed a first episode of psychosis during follow-up was small. However, the mean follow-up period was 17 months, and most transitions to psychosis occur within the first 36 months (
40), and some even later (
41). Thus, it is possible that more subjects in the present sample will develop psychosis as the follow-up period is extended.