Accumulating evidence from epidemiological (
1–
3) as well as retrospective and prospective clinical studies (
4–
11) suggests that patients with major depressive disorder commonly present with manic symptoms below the threshold for hypomania (mixed features). Most prevalence estimates range from 25% to 45%, depending in part on how the population studied was defined, including the number and duration of manic symptoms. While rigorous comparisons are not available, a substantial body of evidence suggests that major depressive disorder with mixed features, in contrast to pure major depression, is characterized by greater illness severity (
11,
12), increased frequency of depressive episode recurrence (
2,
11), increased risk of suicide attempt (
2,
8,
12–
14), comorbid anxiety disorder (
2,
8,
12) and substance abuse (
2,
8,
12), greater functional disability (
2,
12,
15), and poorer prognosis (
12,
14). Given developments in the understanding of the prevalence of this clinical presentation, as well as its distinct course and prognosis, DSM-5 incorporated a new “mixed features” specifier that may be used to recognize the presence of subthreshold symptoms of the opposite pole in patients presenting with either depressive or manic episodes.
No controlled trials to date have investigated the efficacy of any psychotropic agent in the treatment of major depressive disorder with mixed features. While standard antidepressants are widely used in the treatment of major depressive disorder, the efficacy and safety of these agents in patients with mixed features has not been established and is not well understood. However, multiple clinical reports suggest that standard antidepressants may be ineffective for this condition and may be associated with potential treatment-related complications, including suicidal ideation and behavior, manic switch, agitation, and impulsivity (
14,
16–
19). Although atypical antipsychotic agents and mood stabilizers have been suggested as treatment alternatives (
20,
21), there is little experimental evidence to support these clinical recommendations. Given the limitations of current treatment approaches, as well as the complex course and poor outcomes associated with this form of major depressive disorder, there is a pressing need for evidence-based treatments for this condition.
Lurasidone is an atypical antipsychotic agent with high affinity for D
2, 5-HT
2A, and 5-HT
7 receptors (Ki=1 nM, 0.5 nM, and 0.495 nM, respectively) (
22). In animal models, the antidepressant effect of lurasidone has been shown to be mediated in part by antagonist activity at the 5-HT
7 receptor (
23,
24). Atypical antipsychotics have been shown to have mood-stabilizing properties, and selected agents have demonstrated antidepressant efficacy in bipolar depression (
20). Lurasidone has demonstrated efficacy in the treatment of bipolar depression, both as a monotherapy and as an adjunctive therapy with lithium or valproate (
25,
26). Given this range of clinical effects, we hypothesized that lurasidone may be useful for the treatment of mixed forms of major depression.
The purpose of this study was to evaluate the efficacy and safety of lurasidone for the treatment of patients with major depressive disorder presenting with subthreshold hypomanic symptoms (mixed features).
Method
Patients
This multiregional study enrolled outpatients 18–75 years of age with a diagnosis of major depressive disorder based on DSM-IV-TR criteria, which was confirmed with the Structured Clinical Interview for DSM-IV Disorders–Clinical Trial version, modified to record the presence of mixed symptoms (
27) and administered by an experienced and qualified rater. Patients were required to have a current major depressive episode, with a score ≥26 on the Montgomery-Åsberg Depression Rating Scale (MADRS) (
28) at both screening and baseline visits. In addition, patients were required to have two or three of the following manic symptoms, on most days for at least 2 weeks prior to screening: elevated or expansive mood, inflated self-esteem or grandiosity, more talkative than usual or pressure to keep talking, flight of ideas or racing thoughts, increased energy, increased or excessive involvement in activities with a high potential for negative consequences, and decreased need for sleep. Patients presenting with irritability, distractibility, and psychomotor agitation could be enrolled; however, consistent with DSM-5, these nonspecific symptoms were not included in the list of eligible manic symptoms required for study entry. External clinical reviewers verified the diagnoses of all study participants, based on audio recordings of diagnostic interviews conducted by site-based investigators.
The study was approved by an institutional review board at each investigational site and was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use’s Good Clinical Practice guidelines, and with the ethical principles of the Declaration of Helsinki. An independent data and safety monitoring board reviewed and monitored patient data throughout the study.
Study Design
This randomized, double-blind, placebo-controlled, flexible-dose study enrolled a total of 211 patients at 18 sites in the United States (N=62 patients) and 26 sites in Europe (N=149 patients) between September 2011 and October 2014.
After a washout period of at least 3 days, patients were randomly assigned, in a 1:1 ratio via an interactive voice/web response system, to receive 6 weeks of treatment with lurasidone or placebo. Study medication was provided in blister packs as identically matched tablets containing placebo or 20 mg or 40 mg of lurasidone. A central randomization center used a computer-generated list of random numbers to allocate study treatments. None of the investigators, study staff or patients had access to the randomization codes or list. Study medication was taken once daily in the evening with a meal or within 30 minutes after eating. Patients assigned to receive lurasidone were treated with 20 mg/day for days 1–7. Patients were dosed flexibly, in the range of 20–60 mg/day, starting on day 8.
Concomitant Medications
Treatment with anticholinergic agents, propranolol, or amantadine was permitted as needed (but not prophylactically) for movement disorders. Lorazepam, temazepam, or zolpidem (or their equivalent) were permitted during screening and weeks 1–3, as needed for anxiety or insomnia.
Efficacy Assessments
The primary efficacy endpoint was mean change from baseline to week 6 in MADRS total score. The key secondary efficacy endpoint (corrected for multiplicity) was mean change from baseline to week 6 in Clinical Global Impressions severity subscale score (CGI-S), which rates overall illness severity on a 7-point scale (
29). Standard a priori criteria were employed for treatment response (≥50% reduction from baseline in MADRS score) and remission (MADRS score ≤12). Additional secondary efficacy assessments included the Young Mania Rating Scale (YMRS) (
30), the Hamilton Anxiety Rating Scale (HAM-A) (
31), and the Sheehan Disability Scale (
32).
Safety and Tolerability Evaluations
Safety and tolerability assessments included incidence and severity of adverse events; the Simpson-Angus Rating Scale, the Abnormal Involuntary Movement Scale (AIMS), and the Barnes Akathisia Rating Scale to evaluate movement disorders; and vital signs, laboratory tests, 12-lead ECG, and physical examination. Suicidal ideation and behavior were assessed using the Columbia–Suicide Severity Rating Scale (
33). Treatment-emergent mania was defined, a priori, as a YMRS score ≥16 on any two consecutive assessments or at the final assessment, or an adverse event of mania or hypomania. Sexual functioning was assessed using the 14-item Changes in Sexual Functioning Questionnaire (
34); total scores ≤47 for men and ≤41 for women indicate sexual dysfunction, and higher scores are associated with adequate sexual functioning.
Statistical Analysis
The safety population included all patients who were randomized and received at least one dose of study medication. The intent-to-treat population consisted of randomized patients who received at least one dose of study medication and had at least one postbaseline MADRS or CGI-S assessment. The primary (MADRS) and key secondary (CGI-S) efficacy endpoints, as well as the YMRS, were assessed using a mixed model for repeated-measures analysis including fixed effects for treatment, visit, and pooled center; baseline score as a covariate; and a treatment-by-visit interaction term. An unstructured covariance matrix was used for within-patient correlation. In the analysis of the MADRS and CGI-S, a sequential testing procedure was used to control overall type I error at 5%.
Changes from baseline in secondary efficacy measures (HAM-A, Sheehan Disability Scale) were evaluated using an analysis of covariance (ANCOVA) model (including terms for treatment, pooled center, and baseline score) using last observation carried forward. Inferential analysis of secondary efficacy endpoints was not corrected for multiplicity. Effect sizes (Cohen’s d) were calculated as the least squares mean difference in the change score divided by the pooled standard deviation. The number needed to treat to achieve response (based on prespecified MADRS criteria) was calculated by assessing the reciprocal of the difference in responder rates in the lurasidone and placebo groups (
35); the number needed to harm for selected adverse events was calculated by assessing the reciprocal of the difference in adverse effect rates for the lurasidone and placebo groups (
35). Likelihood of being helped or harmed (the ratio of number needed to harm to number needed to treat) was calculated to illustrate trade-offs between benefits (response) and harms (incidence of individual adverse events and of an increase ≥7% in body weight [
35]).
The estimated sample size of 100 patients per treatment group (adjusted for attrition) was determined based on a two-sample t test and was powered at 80% to detect a 4.5-point difference at week 6 in MADRS change scores for lurasidone compared with placebo, with a common standard deviation of 10.
Results
Baseline Characteristics and Patient Disposition
A total of 327 patients were screened, of whom 211 (64.5%) were randomly assigned to 6 weeks of double-blind treatment (see Figure S1 in the data supplement that accompanies the online edition of this article). Of these, 209 patients (lurasidone, N=109; placebo, N=100) received at least one dose of study medication and comprised the safety population, of whom 208 comprised the intent-to-treat population used in all efficacy analyses. Study completion rates were high in both the lurasidone group (93.6%) and the placebo group (85.3%) (see Figure S1). The mean daily dose of lurasidone during the study was 36.2 mg; the modal daily dose of lurasidone was 20 mg for 32% of patients, 40 mg for 29%, and 60 mg for 39%.
Baseline demographic and clinical characteristics were similar for the lurasidone and placebo groups (
Table 1). The mean reported lifetime number of prior major depressive episodes was 4.3. The mean duration of the index depressive episode was 3.5 months, and of subthreshold hypomanic symptoms (mixed features) prior to screening, 2.6 months. A total of two protocol-specified manic symptoms were present in 130 patients (62.5%), and three symptoms in 78 patients (37.5%). The proportion of patients reporting specific manic symptoms at baseline was as follows: flight of ideas/racing thoughts, 66.8%; pressured speech, 61.1%; decreased need for sleep, 40.8%; increased energy/activity, 28.0%; elevated/expansive mood, 18.0%; increased/excessive involvement in pleasurable activities, 15.6%; and inflated self-esteem/grandiosity, 6.6%. The “nonspecific” symptoms of irritability, distractibility, and psychomotor agitation were reported at baseline by 57.3%, 59.2%, and 36.5% of patients, respectively.
Efficacy
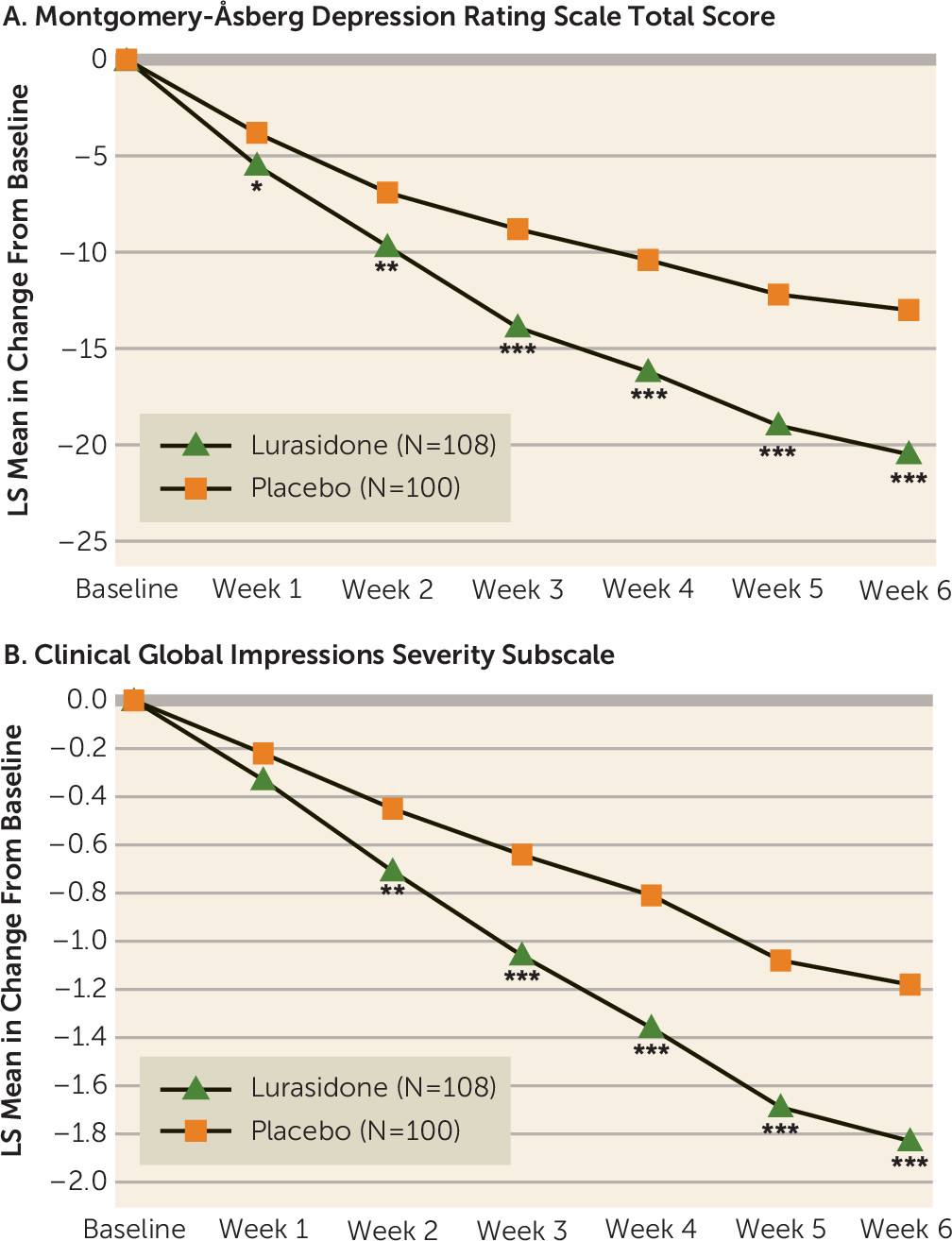
The least squares mean change from baseline to week 6 in the MADRS total score (primary endpoint) was significantly greater for lurasidone compared with placebo (−20.5 and −13.0, respectively; p<0.001; effect size, 0.80) (
Table 2). Least squares mean change at week 6 in the CGI-S score (key secondary endpoint) was also significantly greater for lurasidone compared with placebo (−1.8 and −1.2, respectively; p<0.001; effect size, 0.60) (
Table 2). Statistical superiority compared with placebo was observed from week 1 through week 6 for the MADRS and from week 2 though week 6 for the CGI-S (
Figure 1).
With respect to the MADRS, there was no statistically significant treatment interaction with gender, age, or geographic region. At week 6, significantly greater improvement in the MADRS total score was observed in the two geographic regions where the study was conducted, the United States (N=59; p<0.01; effect size, 0.78) and Europe (N=149; p<0.001; effect size, 0.92).
A significantly higher proportion of patients in the lurasidone group than in the placebo group met a priori response and remission criteria at week 6 (response: 64.8% compared with 30.0%; p<0.001; number needed to treat=3 [last observation carried forward]; remission: 49.1% compared with 23.0%; p<0.001; number needed to treat=4 [last observation carried forward]).
Lurasidone was associated with significantly greater least squares mean change at week 6 compared with placebo on the YMRS (−7.0 compared with −4.9; p<0.001; last observation carried forward), the HAM-A (−9.9 compared with −5.4; p<0.001), and the Sheehan Disability Scale (−11.2 compared with −6.4; p<0.001; last observation carried forward) (
Table 2).
Change in MADRS score was assessed on an exploratory basis for patients with two compared with three protocol-specified manic symptoms at study baseline. Least squares mean change in MADRS score from baseline to week 6 was significantly greater with lurasidone than placebo for both patient subgroups. For patients with two manic symptoms, the change in MADRS score with lurasidone was −20.7, compared with −11.5 with placebo (p<0.001; effect size, 0.98); for patients with three manic symptoms, the change in MADRS score with lurasidone was −20.0 compared with −15.3 with placebo (p=0.038; effect size, 0.50).
Safety
Treatment-emergent adverse events reported with an incidence ≥2% in the lurasidone group, and more frequently than in the placebo group, were nausea, somnolence, dizziness, akathisia, abdominal discomfort, dry mouth, and parkinsonism (
Table 3). Number needed to harm was 23 for the two treatment-emergent adverse events with an incidence >5% (nausea and somnolence). There were no deaths in the study, and no treatment-related serious adverse events. The proportion of patients who discontinued because of treatment-emergent adverse events was 2.8% in the lurasidone group and 5.0% in the placebo group (see Figure S1 in the online
data supplement).
The incidence of extrapyramidal symptom-related adverse events was 2.8% in the lurasidone group and 1.0% in the placebo group. There were minimal changes in scores on the movement disorder assessments. Only 1.8% of the lurasidone group received anticholinergic medication, and none in the placebo group. The proportion of patients treated with anxiolytics was 9.2% for the lurasidone group and 12.0% for the placebo group; the proportion treated with sedatives and hypnotics was 10.1% for the lurasidone group and 11.0% for the placebo group.
Week 6 change in weight and BMI was minimal for both treatment groups (
Table 4). The proportion of patients with an increase ≥7% in body weight at week 6 was 1.9% in the lurasidone group and 1.0% in the placebo group (number needed to harm=112). Mean week 6 change in waist circumference was similar for the two groups (+0.18 cm compared with +0.05 cm; last-observation-carried-forward endpoint). There were no clinically meaningful between-group differences in laboratory measures of lipids, glycemic indices, prolactin levels, or ECG parameters (
Table 4).
Based on the Columbia–Suicide Severity Rating Scale, the proportions of patients with treatment-emergent suicidal ideation or behavior in the lurasidone and placebo groups (5.5% and 7.0%, respectively) were similar, as were the proportions with treatment-emergent mania (2.8% and 5.0%, respectively).
The mean score on the Changes in Sexual Functioning Questionnaire at baseline was 35.4 for the lurasidone group and 34.1 for the placebo group. Patients in the lurasidone group were found to have significant improvement in sexual functioning at endpoint compared with those in the placebo group according to Changes in Sexual Functioning Questionnaire score (+5.1 compared with +3.1; p<0.05).
For lurasidone treatment, the likelihood of being helped or harmed was >1 (indicating that benefit from treatment [MADRS response] was more likely than harm) for nausea (likelihood of being helped or harmed, 8), somnolence (likelihood of being helped or harmed, 8), and increase of ≥7% in body weight (likelihood of being helped or harmed, 38).
Discussion
This is, to our knowledge, the first placebo-controlled clinical trial that included patients with major depressive disorder associated with subthreshold hypomanic symptoms (mixed features). Lurasidone significantly improved depressive symptoms compared with placebo, based on both the primary MADRS assessment and the CGI-S assessment of overall illness severity. Significantly greater improvement in favor of lurasidone compared with placebo was observed from weeks 1 through 6 on the MADRS, and from weeks 2 through 6 on the CGI-S, with effect sizes in the moderate to large range for both measures at endpoint. Manic symptoms were also significantly improved in the lurasidone group, suggesting efficacy across the range of core mood symptoms associated with this disorder. In addition, treatment with lurasidone was associated with significant improvement both in anxiety symptoms and in patient-reported functional impairment.
The mixed-features variant is a severe form of major depression characterized by the presence of several manic symptoms below the threshold for hypomania in patients with no history of mania or hypomania. Since patients with this condition have not been systematically studied in controlled trials, little evidence exists to guide treatment selection. This placebo-controlled study provides the first rigorous evidence that an atypical antipsychotic agent, lurasidone, may be an effective and safe treatment for this condition.
The diagnostic classification of major depression with mixed features has been a subject of much debate (
36). Shared clinical characteristics, as well as similarity to bipolar patients in age at onset, family history, and course of illness (
2,
8,
12,
36), suggest that patients with mixed features represent an intermediate phenotype between major depressive disorder and bipolar depression. Mixed features associated with a major depressive episode increase the risk for the development of bipolar disorder, and the presence of subthreshold manic symptoms appears to reduce treatment responsiveness to standard antidepressants, further substantiating this impression (
8,
36).
We defined mixed features in this study as the presence of two or three protocol-specified manic symptoms for at least 2 weeks prior to screening. The permissible number of manic symptoms was limited to three to reduce the likelihood that patients with undiagnosed bipolar disorder would be enrolled in the study. The finding that treatment effect size was somewhat smaller in patients with three manic symptoms at baseline is consistent with previous reports that patients with admixtures of mood symptoms of opposite polarity (e.g., mixed mania) may show reduced treatment responsiveness compared with those with nonmixed forms of depression or mania (
35,
36). However, further research is needed to confirm this exploratory finding in this patient population.
The presence of subthreshold hypomanic features has been shown to contribute to the heterogeneity of major depressive disorder and may add to the difficulty of achieving diagnostic reliability (
8). Increased clinical efforts are needed to identify the presence of mixed features in depressed patients, both to improve case ascertainment and because the presence of as few as one or two manic symptoms has been associated with an increased risk for episode recurrence, suicide attempt, substance abuse, and poor antidepressant response (
2,
8,
11,
12,
14).
The findings reported here confirm that this patient population can be identified in outpatient and community settings and provide further validation of the DSM-5 mixed features specifier in terms of its potential to better characterize symptoms associated with major depressive episodes, as well as to predict treatment response. The manic symptoms most commonly reported at study entry (racing thoughts and pressured speech) are consistent with reports in previous clinical populations (
15,
17,
37). Therefore, the presence of these manic symptoms during a major depressive episode should be regarded as suggestive of the mixed-features diagnosis, and inquiry should be made to assess the presence of additional symptoms. This study also confirms previous findings that irritability, distractibility, and psychomotor agitation (nonspecific symptoms that may be due to depression or mania) occur at relatively high rates in patients with mixed forms of major depression (
38).
Lurasidone previously demonstrated efficacy in bipolar depression (
25,
26), and the positive results of the present study now extend those findings to the treatment of a related disorder, major depressive disorder presenting with mixed features. The lurasidone dosage range utilized in this study (20–60 mg/day) partly overlapped with the range demonstrated to be effective for bipolar depression (20–120 mg/day). A dose-response relationship was not observed for lurasidone in a previous bipolar depression monotherapy study that evaluated lower and higher dosage ranges (
25). Whether higher lurasidone dosages might provide additional efficacy in patients with mixed forms of major depression is not known.
The relatively low dosages of lurasidone utilized in this study suggest that the pharmacology of lurasidone in major depression with mixed features involves the serotonin and dopamine neuroreceptors to which lurasidone binds with high affinity (5-HT
2A, 5-HT
7, and D
2 receptors) (
22). Given the current preclinical understanding of the role of the 5-HT
7 receptor in mediating antidepressant effects (
23,
24), antagonist effects at this receptor may account for much of the observed treatment effect.
Use of lower dosages of lurasidone may have contributed to its tolerability in this study. The majority of adverse events associated with lurasidone treatment were reported as mild or moderate in severity (97.2%), and we observed low rates of study discontinuation for adverse events (2.8%) or for any reason (6.4%). Notably, rates of treatment-emergent mania and of suicidal ideation or behavior were comparable for lurasidone and placebo.
The number needed to treat to achieve a MADRS-defined response with lurasidone was 3. The number needed to harm for the two most frequently reported adverse events (nausea and somnolence) was 8, and for an increase ≥7% in body weight, it was 38. Thus, the likelihood of being helped or harmed (the ratio of number needed to harm to number needed to treat) was consistently >1, indicating that benefit from treatment with lurasidone was more likely than harm, when contrasting treatment response and specific adverse events. These results support the benefit-risk profile of lurasidone in the population studied, and they are consistent with similar findings in patients with bipolar depression (
35,
39).
This study has several limitations. First, patients were eligible for study entry with either two or three manic symptoms, while DSM-5 requires at least three manic symptoms to meet criteria for the mixed features specifier. DSM-5 criteria also require manic or hypomanic symptoms to be present nearly every day during the majority of days of a major depressive episode, while in this study they were required to have occurred on most days for at least 2 weeks prior to screening. We also note that while the long-term safety of lurasidone has been established in a variety of patient populations, longer-term efficacy and safety have not been specifically studied in this patient population.
In summary, there is a pressing need for evidence-based treatments of major depressive disorder presenting with subthreshold hypomanic symptoms (mixed features), especially given its complex course and associated morbidity. Lurasidone was found to be an efficacious treatment in this patient population, with improvements observed in depressive and subthreshold hypomanic symptoms, anxiety symptoms, and functional impairment. Treatment with lurasidone was well tolerated, with a favorable benefit-risk profile in this difficult-to-treat clinical population. Further investigation is needed to determine whether these findings are applicable to other agents in the atypical antipsychotic class.
Acknowledgments
The authors thank the study participants and the study investigators: in Great Britain, Drs. A. Asher and R. Balasubramanya; in Russia, Drs. N. Neznanov, M. Burdukovsky, P. Muchnik, A. Parashchenko, Y. Barylnik, V. Savitskaya, and I. Boyev; in Serbia, Drs. S. Djukic Dejanovic, J. Petrovic, D. Lecic Tosevski, J. Nikolic Popovic, V. Diligenski, G. Grbesa, T. Papic, S. Jekic Tanovic, A. Miljatovic, and V. Savic; in Ukraine, Drs. K. Veselin, S. Kazakova, O. Serebrenikova, V. Pidkorytov, K. Zakal, A. Voloshchuk, G. Ziberblat, and S. Moroz; and in the United States, Drs. S. Atkinson, P. Bhatia, E.S. Brown, A. Cutler, M. Downing, B. D’Souza, D. Gruener, J. Heussy, A. Patkar, L. Chung, T. Suppes, D. Walling, I. Yuryev-Golger, G. Mattingly, J. Breving, B.L. Barnhart, V. Mehra, and H. Logue.