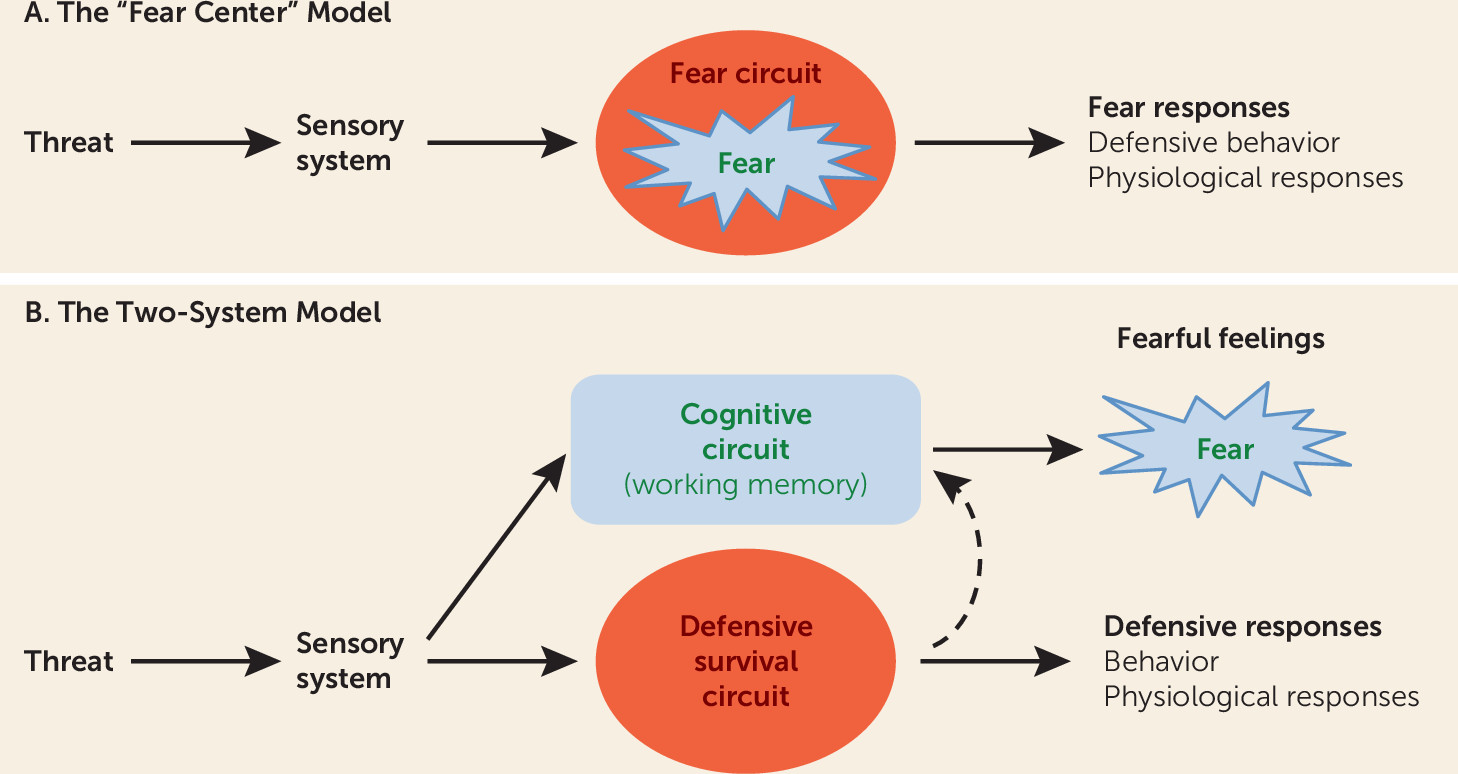
It has long been assumed that an innate “fear system” exists in the mammalian brain and that this system, in the presence of a threat, generates both the conscious feeling of “fear” and the behavioral and physiological responses typical of such experiences (
Figure 1A). We propose instead a “two systems” framework, with one set of circuits for generating conscious feelings and a second set for controlling behavioral and physiological responses to threats (
Figure 1B). The first system primarily involves cortical areas, and the second mostly involves subcortical regions, such as the amygdala, although certain cortical areas interact with and regulate processing in these regions. While the first system generates conscious feelings, the second largely operates nonconsciously. Conflation of these circuits and their functions has hampered clinical extension of basic research. While the actual circuitry is considerably more complex than implied by the two-system label, the framework represents a useful heuristic around which to restructure translational efforts to achieve a deeper, more nuanced understanding than currently exists about how brain mechanisms give rise to normal and pathological feelings of fear and anxiety and the behavioral and physiological symptoms that accompany these subjective experiences.
A Note on Terminology
The terms “fear” and “anxiety” are used in many ways. Consider fear. Most often it refers to a subjective state, a feeling that one experiences when threatened. However, it also describes behaviors, such as facial expressions, freezing, flight, and avoidance, as well as physiological changes that accompany such behaviors. Using the same term for both subjective states and objective responses implies that they are entwined in a common neural circuit. We and others argue that this assumption is incorrect—that the different processes referred to by the term “fear” reflect different mechanisms rather than operations of one “fear center” or “fear circuit” (
3–
9).
Because precise terminology is essential for scientific progress, we propose that the use of mental state terms, like fear and anxiety, be limited to their primary, as opposed to their extended, meanings, that is, to mental states—subjective feelings of fear and anxiety. We further propose that these mental state terms be avoided when referring to behavioral and physiological responses that also may occur when one feels fearful or anxious. Accordingly, we refer to brain circuits that detect and respond to threats as defensive circuits, to behaviors that occur in response to threats as defensive behaviors, and to peripheral changes in physiology that support defensive behaviors as defensive physiological adjustments.
Confusion also results from interchangeable use of the terms fear and anxiety. To avoid such confusion, we propose using a common distinction consistently—that the mental state term fear be used to describe feelings that occur when the source of harm, the threat, is either immediate or imminent, and anxiety be used to describe feelings that occur when the source of harm is uncertain or is distal in space or time.
The Innate Fear Circuit View
Fear is often said to be an innate function of subcortical brain areas. This view stems from the idea that humans inherited from animals certain basic, universally expressed emotions (
10,
11), often described as products of the brain’s so-called limbic system (
12,
13). For example, fear is often said to be a product of the limbic area called the amygdala, which is frequently said to be a “fear center,” or, in more contemporary terminology, the hub of a “fear circuit” (
13–
22).
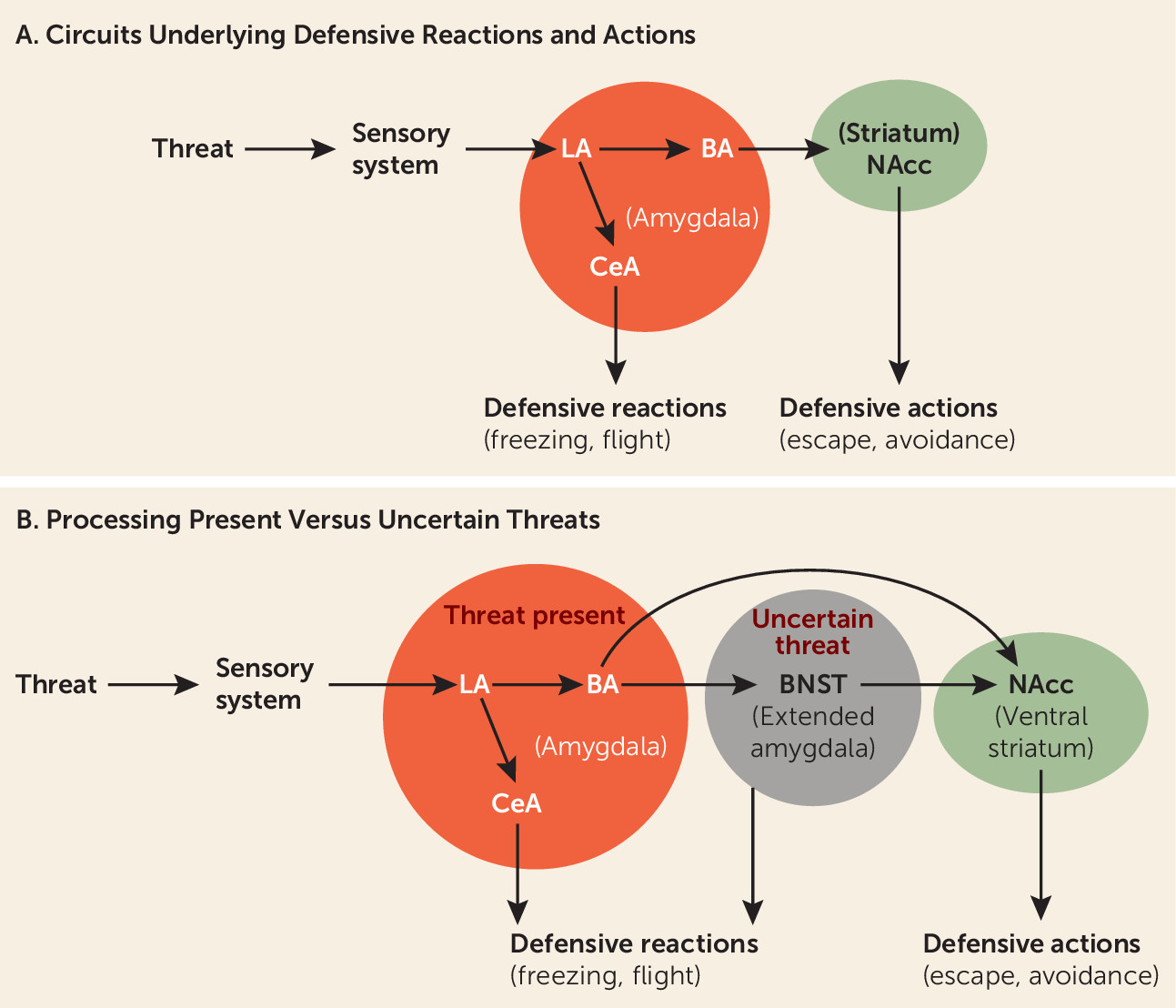
An immediately present threat activates the lateral nucleus of the amygdala, which by way of connections to the central nucleus of the amygdala initiates the expression of defensive behavioral reactions, such as freezing, and supports defensive physiological reactions (
Figure 2A). And through connections from the lateral amygdala to the basal amygdala, and from there to the nucleus accumbens, defensive actions such as avoidance are controlled (
5,
23). Although key components of this circuitry are subcortical, the ability of the circuits to control defense reactions and actions is modulated by certain cortical areas. For example, the extinction of defensive responses elicited by learned threats is regulated by connections from the ventral medial prefrontal cortex and hippocampus to the amygdala (
24–
27).
Findings in humans are consistent with the animal data. Thus, people with amygdala damage fail to exhibit bodily reactions to threats (
28,
29). Furthermore, functional imaging studies show that threats activate the amygdala in healthy people (
28,
30) and induce exaggerated amygdala activation in patients with anxiety disorders (
31–
34). In addition, the nucleus accumbens has been implicated in defensive actions such as avoidance in humans (
35,
36). Medial cortical areas down-regulate the amygdala in healthy humans (
25,
37), and this capacity is weakened in people with anxiety disorders (
26,
27). Such findings are commonly viewed as support for the idea that the amygdala is a hub in an evolutionary conserved fear circuit that, in the presence of a threat, controls both feelings of fear and behavioral and physiological sequelae.
The assumption that in the presence of a threat the same circuit controls conscious feelings of fear and behavioral and physiological responses is expressed most forcefully by Panksepp (
13,
16). He notes that “fear is an aversive state of mind” and that the major driving force for this “subjective
component of fear seems to be … a subcortical FEAR system” (
16). Since both the behavioral and physiological responses and the subjective feelings of fear elicited by a threat are viewed as products of the same circuit, it should be possible, according to Panksepp, to identify the “feeling circuit” in humans by studying behavioral and physiological responses in animals or humans. But if, as we have argued, different circuits underlie the two consequences of threat detection, studying behavioral or physiological responses will not reveal the circuits responsible for feelings.
Just as findings demonstrating that the amygdala detects and controls behavioral and physiological responses to immediate threats have supported views of the amygdala as fear-circuit hub, other findings, about responses to uncertain threats, have led to a view of the circuitry of anxiety. Thus, in recent years, animal research has suggested the bed nucleus of the stria terminalis (BNST) is engaged when threats are uncertain (
38,
39), resulting in behavioral inhibition and risk assessment (
40) (
Figure 2B). Imaging studies in healthy humans (
41) and in humans with anxiety disorders confirm the contribution of the BNST in processing uncertainty (
42,
43). The BNST has thus come to be for anxiety what the amygdala is for fear—a circuit hub out of which anxious feelings emerge.
Proponents of subcortical explanations do not all mean the same thing when they use the terms
fear and
anxiety. For a number of contemporary neuroscientists, emotions are not subjective experiences but central physiological states (“central states” for short) that intervene between trigger stimuli and behavioral and physiological responses (
14,
17–
19,
40,
44,
45). A strong version of the central state view of fear is expressed by Fanselow and colleagues (
45). They argue that a goal of science should be to “replace inaccurate subjective explanations … with more scientifically grounded explanations.” This approach ignores subjective states to avoid the scientific problems that result from attributing subjective experiences to animals. But it does so at the expense of making subjective experience off-limits as a scientific topic in humans. This is a serious shortcoming of any approach attempting to translate animal research to human clinical problems. Subjective experiences of fear or anxiety typically are the problems that lead people to seek help; moreover, therapies are judged as successful largely on the basis of their capacity to change these experiences. Softer versions of the central state view that allow the central state to contribute to both feelings of fear and bodily responses (
19) are essentially “fear circuit” views and are subject to the criticisms mentioned above.
Problems With the Subcortical Fear and Anxiety Center/Circuit Views
That a brain area, like the amygdala, controls behavioral and physiological responses to threats does not mean that the experience of fear arises from this brain area. In other words, it is an assumption, not a fact, that both consequences of threat detection are products of a single circuit. And this assumption is contradicted by several sets of findings.
First, it has long been known that subjective experiences of fear and anxiety do not correlate well with measures of behavioral and physiological responses (
46–
48). This should not be the case if the same circuit controls all these consequences of threat detection. Second, patients with amygdala damage still can feel fear, panic, and pain (
49–
51). Earlier reports of diminished feelings following amygdala damage (
52) may reflect the elimination of the indirect consequences of amygdala activity on feelings (see below). Third, threats presented subliminally increase amygdala activity and trigger peripheral physiological responses, even when one remains unaware of the stimulus and lacks feelings of fear (
28,
30,
53–
59). Fourth, “blindsight” patients, who, as a result of damage to the right visual cortex, lose the ability to consciously experience stimuli presented to their left visual hemifield (
60), nevertheless exhibit threat-elicited amygdala activity and defensive behavioral and physiological responses, all in the absence of conscious awareness of the stimulus and without feeling fear (
61–
63). All four sets of findings dissociate the circuitry underlying feelings from circuitry underlying defensive behavior and physiologic responding. How can this be if the amygdala is a fear center?
We thus argue that the amygdala is
not itself responsible for the experience of fear. Its job can be more appropriately viewed as detecting and responding to present or imminent threats. The amygdala contributes to fear indirectly but is not an innate fear center out of which fear percolates. And the BNST is not itself responsible for the experience of anxiety, but instead is a key part of an innate defense circuit that detects and processes uncertain threats. The BNST contributes to anxiety indirectly in the way that the amygdala contributes indirectly to fear—consequences of its activation generate signals that modulate circuits that are directly responsible for subjective feelings of fear and anxiety (
5).
The Emergence of Conscious Experience From Cortical Circuits
Significant progress has been made in neuroscience research on the cognitive and neural underpinnings of subjective experiences (
60,
64–
72). This work assumes that conscious experience is cognitively derived from nonconscious processes that allow cortical regions to rerepresent lower-order information, and that this rerepresentation enables conscious awareness of nonconscious processing about external stimuli.
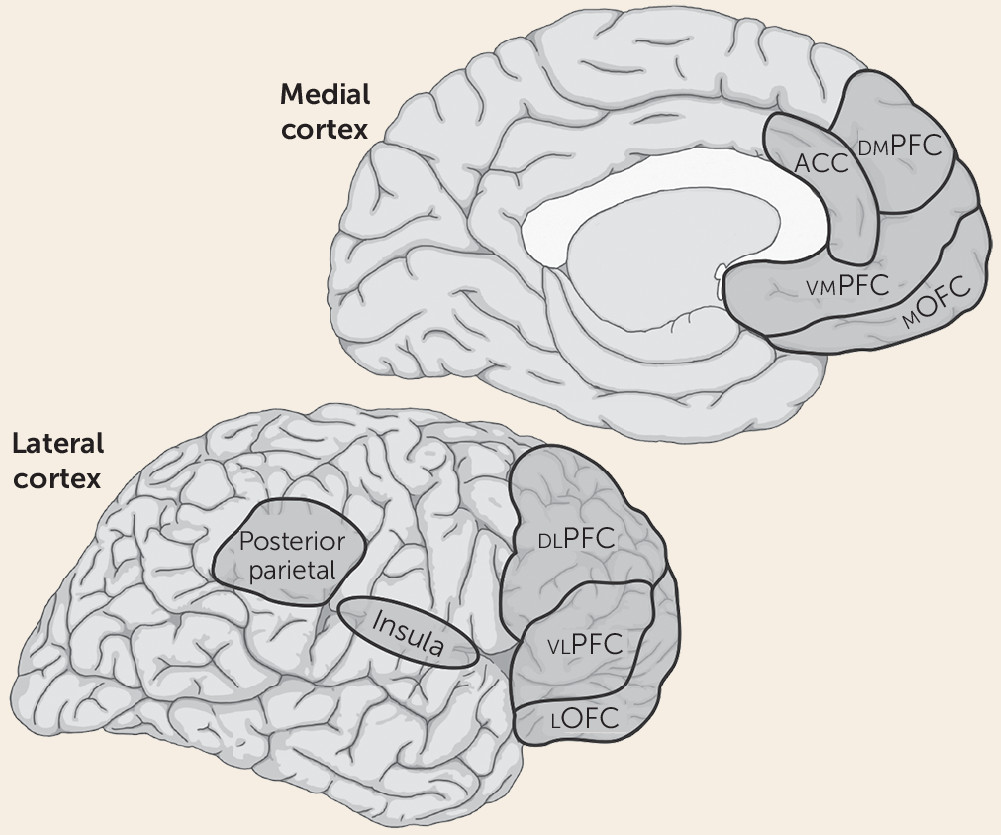
We propose that subjective feelings of fear or anxiety are not products of subcortical circuits underlying defensive responses, but instead depend on the same circuits that underlie any other form of conscious experience—namely, circuits in the so-called higher-order association cortex that are responsible for cognitive processes such as attention and working memory (
Figure 3). Included are areas of the lateral and medial prefrontal cortex, as well as the parietal neocortex (
73–
77). The lateral prefrontal areas may be especially important since they have been most consistently implicated in conscious awareness (see below), are interconnected with the other key cortical areas, are particularly well developed in primates, and have unique attributes in humans (
78–
83). The insula, another frontal region, has been implicated in the conscious experience of somatic sensations (
84–
86) and may be particularly relevant to threats signaled by perturbations in the organism’s physiologic milieu and in forms of anxiety initiated by interoceptive stimuli (
87,
88).
When one is aware of a visual stimulus, prefrontal and parietal circuits are engaged, and when awareness degrades, the circuits are not engaged (
64,
89–
91). Two leading theories propose two different ways that this neural architecture gives rise to conscious experience. In global workspace theory, subjective experience emerges through widely distributed reentrant circuitry, with prefrontal areas playing an especially prominent role (
64,
65,
92). In higher-order theory, subjective experience arises from a more delimited circuitry, especially involving a prefrontal hub, which supports thoughts about lower-order information (
67,
93,
94).
While attention, working memory, and their underlying circuits support consciousness, working memory can be engaged without generating conscious content (
71,
92,
95–
97). An additional layer of cognitive representation, likely also involving the frontal cortex, is thus required beyond nonconscious representation in working memory (
94). Differentiating neural features of cognitive processing that do and do not result in introspective awareness is a significant challenge and a major focus of current research.
Consistent with findings described so far, when participants in a brain imaging study are conscious of a visual threat, prefrontal and parietal areas are active, but when awareness is degraded, prefrontal and parietal areas are not (
28,
30,
53–
59). Importantly, as also noted above, in such studies, the amygdala is engaged even when people are not consciously aware of the threat and do not report feeling fear. This has two important implications. First, amygdala processing is dissociable from conscious awareness of threat. Second, conscious awareness of threats comes about in the same way as the conscious awareness of nonemotional stimuli.
We thus propose that emotional and nonemotional states of consciousness both emerge from cortical consciousness networks. The difference between an emotional and a nonemotional state of consciousness, in this view, reflects different kinds of inputs to the cortical consciousness network in the two kinds of states: the inputs required to feel an emotion elicited by a threat are more elaborate than those required to see a nonthreatening stimulus. While the feeling of fear thus does not arise directly from subcortical circuits that control behavioral and physiological responses to threats, these circuits indirectly contribute to the feeling of fear by generating responses, such as brain and body arousal, which can affect working memory function.
Although feelings of fear and anxiety are most often discussed in terms of circuits that evolved for predatory defense, this view is too narrow. We can also be afraid or anxious about activity related to other survival circuits (
3–
5), including circuits that signal low energy supplies (fear of starvation), fluid imbalance (fear of dehydration), or hypothermia (fear of freezing to death). In each example, fear/anxiety reflects awareness of a potential for harm, occurring when one cognitively monitors and interprets signals from the brain and/or body, and integrates these signals with information about the external situation. But there is more. Humans can also be fearful or anxious in relation to existential concerns, such as not leading a meaningful life and the eventuality of death. A scientific account of “fear” and “anxiety” has to accommodate all the various ways that fear and anxiety can manifest within and between individuals. The two-system view, which treats fear and anxiety as cognitively generated conscious experiences, accomplishes this.
Separating circuits underlying conscious feelings and response control also makes it easier to understand the role language and culture play in the shaping of experience (
98–
101). In English alone, there are more than three dozen words for gradations of fear and anxiety, reflecting the importance of these states to humans (
102). Language enables symbolic representation of emotions like fear and anxiety without actual exposure to danger (
103,
104). In certain circumstances, this can help keep us safe, but it can also become a vehicle for excessive rumination and worry.
The two-system view also may explain why infants react emotionally long before they report emotions (
105). Interestingly, the defense circuitry in infants is not simply an immature version of the adult circuitry (
106). Developmental changes in linguistic capacity allow categorization of experience and may affect the way fear is expressed at different points in early life: children report different fears than adolescents, who report different fears than adults (
107,
108). Understanding these changes requires a deeper understanding of brain development (
109). As discussed later, this also affects perspectives on treatment.
Whatever degree of self-awareness and conscious experience is possible without language, it is clear that language changes the self-and-consciousness game in the brain. We are not necessarily suggesting that animals lack conscious experiences, but rather that it is problematic to draw inferences about conscious experiences, and thus problematic to use words like fear and anxiety, when describing animal behavior and physiology. Moreover, because, as noted, defensive behaviors and physiological responding are not reliable indicators of subjective fear in humans, such responses in animals cannot be used to identify circuits responsible for feelings of fear or anxiety in animals or humans.
The two-system framework provides a way to understand what animal research can and cannot tell us about human fear and anxiety without making the impossible-to-evaluate assumption that in animals exposure to threat elicits states that are comparable to what humans call feelings of fear and anxiety. Another important impact of the two-system view is that it helps us understand why efforts to develop medications have not been more effective. If feelings of fear or anxiety are not products of circuits that control defensive behavior, studies of defensive behavior in animals will be of limited value in finding medications that can relieve feelings of fear and anxiety in people. Once this is realized, animal research can be viewed in a more realistic light as being relevant to a particular subset of symptoms in anxiety disorders (those arising from subcortical circuits). While it may never be possible to directly study conscious states in animals, animal research, especially nonhuman primate research, may nevertheless be able to make some indirect contributions by revealing neural underpinnings of cognitive processes, such as attention and working memory, that contribute to conscious experiences, including conscious feelings, in humans.
In sum, being clearer about when research is relevant to feelings of fear and anxiety, as opposed to defensive behavior and physiological responses, should enhance the translational impact of animal research. In particular, it will clarify when these two sets of phenomena show overlapping and distinct associations with clinical variables.
Implications for Stalled Progress in Therapeutics
The two-system framework offers a path forward for finding better medications and psychotherapies. We focus on medication development, given recent discussions about the poor outcome of this effort (
1). In spite of major expenditures over several decades, the results have been disappointing (
2). The pharmaceutical industry has concluded that the probability of discovering new treatments is low (
2,
110), and it has curtailed investment in future psychotropic medication discovery.
To better understand the problem, consider how novel agents are tested for anxiolytic properties. The usual approach is to study effects on the behavioral responses of rodents in challenging situations. For example, rodents tend to avoid brightly lit, open areas where they can be easily seen, captured, and eaten. If a novel compound leads animals to spend more time in open areas or other threatening situations, it becomes a candidate for treating anxiety in people. While new medications have emerged using this strategy, they inconsistently reduce the feelings of fear or anxiety that cause people to seek help.
We argue that the disappointment in the results reflects two faulty assumptions: 1) that a common circuit underlies the expression of defensive responses and feelings of fear when threatened, and 2) that the circuits that contribute to defensive responses in animals can be used to determine how the human brain gives rise to feelings of fear and anxiety.
If, as we argue, feelings of fear and anxiety arise from a different system than do defensive behaviors, medications that make rats or mice less defensive, avoidant, and/or physiologically aroused in challenging situations will not necessarily make people feel less fearful or anxious. Incomplete response to benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), or other anxiolytics in humans, we propose, may reflect the actions of these medications in the two systems. While these compounds can act on both cortical and subcortical brain circuits (
111–
114), the key question is the extent to which the clinical efficacy of a given medication reflects changes in a particular circuit.
In general, clinicians judge whether therapy is succeeding on the basis of the degree to which the patient reports feeling better (
115). The expectation that medications that decrease defense and avoidance in animals should also make people feel less anxiety needs reevaluation, as new medications are not meeting this expectation. This distinction can be characterized as one between the effects of medication on behavioral inhibition on the one hand (
116) and anxious feelings on the other (
5). While behavioral inhibition is often used as if it were synonymous with “anxiety,” the two-system framework treats behavioral inhibition as separable from anxious feelings.
Available research demonstrates the importance of this distinction. For example, efforts generally have failed to translate a replicable pattern of behavioral and physiological findings in rodents over 20 years with corticotropin-releasing hormone (CRH) receptor manipulations (
117), even though CRH antagonists engage the CRH receptor in humans (
118). CRH antagonists reduce defensive behaviors and associated physiological responses in animals, but they result in minimal clinical effect in humans, a finding that is consistent with predictions from the two-system perspective. Future research may clarify the extent to which different medications affect the two systems, as well as the degree to which partial response reflects an effect on behaviors, as opposed to conscious feelings, but this is not known at present.
Medications such as SSRIs and benzodiazepines can make some people feel less anxious. But it is important to ask whether this effect is due specifically to a change in anxiety itself. Might the positive effects on anxiety be part of a general blunting of emotion experience? And given that emotions, in our theory, are cognitively assembled states, to what extent are the effects of medications on anxiety due to changes in cognitive underpinnings, such as alterations in attention and memory (including working and long-term memory), rather than in anxious feelings themselves? If the anxiolytic effects of a medication are due to general emotional blunting or to impaired cognitive processing, the term anxiolytic would be a misnomer as a characterization of its treatment properties. While such indirect changes in anxious feelings are certainly useful clinically, progress in developing improved treatments would be facilitated by recognition of the exact ways in which the effect is achieved.
It is worth considering whether it is even possible to develop a pharmaceutical treatment that would specifically target anxious feelings. Because of the diverse effects of the medications, it may be difficult to do so without producing effects on other phenomena, such as feelings of sadness or worthlessness, or cognitive processes such as attention and memory. Existing medications are blunt tools with broad effects. But even with improved specificity, it may not be possible to alter specific feelings. If fear, anxiety, and other emotions are indeed cognitively assembled states that emerge from information integration, rather than being an innate experience prewired into subcortical neural circuits, there is no fear or anxiety locus to target, and the changes in fear and anxiety may always be at the expense of emotional or cognitive capacities.
Medications that fail to reduce feelings of fear or anxiety may nevertheless have beneficial effects by reining in defensive systems that are overly sensitive and are producing exaggerated defensive reactions, excessive avoidance, hyperarousal, hypervigilance, and so forth. The two-system approach suggests that a detailed understanding of neural circuits and their functions can help set realistic expectations about what medications might achieve.