Genetic studies in humans have identified the neuregulin 3 gene (NRG3) as a candidate risk gene for a range of neurodevelopmental disorders, whereby structural and genetic variations within the gene are associated with developmental delay, attention deficit hyperactivity disorder, bipolar disorder, and schizophrenia (
1–
11). Emerging evidence also suggests a key role for NRG3 in murine cortical development and human neocortical function (
12–
14), and recent rodent studies provide in vivo support for a key neurological role of NRG3, with ontogenic models of altered NRG3 signaling displaying abnormal affective behaviors, such as heightened anxiety, increased impulsivity, and reduced social functioning (
15–
17).
Similar to NRG1, NRG3 undergoes complex alternative splicing and promoter use, generating at least 15 alternative transcripts (
7,
13,
18). Our recent cloning of the gene and transcriptional studies in human brain characterized these isoforms into four “classes” based on exon homology, termed NRG3 classes I–IV (
7). Interestingly, the NRG3 classes appear to display distinct roles in the human brain, with classes II and IV having significantly higher expression in fetal neocortex compared with postnatal neocortex (
7). Despite the emerging evidence for NRG3′s role in neurodevelopment and psychiatric risk, and the potential for the NRG3 classes displaying unique functions, the developmental expression trajectories of NRG3 isoforms in the human brain are unknown.
The NRG3 gene spans 1.2 Mb on human chromosome 10q22-q23 and encodes a transmembrane epidermal growth factor protein belonging to the neuregulin superfamily (
19). Linkage with 10q22–q23 and schizophrenia has been confirmed in several populations (
2,
3,
5), with subsequent fine mapping identifying a 13-kb interval in intron 1 of NRG3 containing polymorphisms in strong linkage disequilibrium (rs10883866, rs10748842, and rs658440), proxied by rs10748842 (
9). Genetic variation at rs10748842 is also associated with behavioral traits in patients, including delusion severity, positive and negative symptom severity, and attentional processes (
6–
9), as well as prefrontal cortical activation during working memory (
12). Moreover, single-nucleotide polymorphisms (SNPs) in NRG3 intron 1 (including rs658440) have recently been shown to be associated with bipolar disorder in a genome-wide association study (GWAS) pathway analysis of psychiatric disorders (
20), suggesting that genetic variation at the NRG3 locus may represent a biological mechanism underlying broader psychiatric risk.
While the mechanistic role of rs10748842 remains to be determined, bioinformatics analyses identify the SNP as central to an ultraconserved genomic element and homeodomain binding module that modifies transcription factor binding (
7,
18). In the human brain, the risk allele (T) is associated with increased prefrontal cortical expression of NRG3 classes II and III in patients with schizophrenia, nonpsychiatric adult control subjects, and the fetal neocortex, highlighting a potential molecular mechanism of risk (
7,
18). Furthermore, classes I and IV are significantly increased in the dorsolateral prefrontal cortex (DLPFC) of schizophrenia patients compared with nonpsychiatric control subjects (
7). Collectively, these human studies suggest that balanced regulation of NRG3 transcription plays an important role in normal brain function and that increased NRG3 isoform expression is pathophysiologically relevant to genetic risk for psychiatric disorders.
Given the association of NRG3 with several disorders of neurodevelopment and emergent data suggesting that genes critical to cortical genesis are involved in the pathology of psychiatric illness (
20–
23), we sought to characterize the temporal trajectories of NRG3 class I–IV expression in the developing human prefrontal cortex, from the second trimester of gestation through adult aging. Furthermore, given the potential genetic overlap between schizophrenia and affective disorders (
24) and the recent identification of NRG3-containing GWAS networks associated with bipolar disorder (
20), we investigated expression of NRG3 classes I–IV in the DLPFC of patients with bipolar disorder or major depressive disorder and examined the effects of rs10748842 genotype (
7). Finally, to determine tissue-specific regulation of NRG3 isoform expression, we compared a comprehensive range of human tissues with brain tissue.
We demonstrate that NRG3 classes I–IV display distinct expression trajectories across neocortical development and aging and that, as in schizophrenia, increased expression of NRG3 is observed in depression and bipolar disorder, in a diagnostic-subclass-specific and genetically regulated manner. Additionally, we present what we believe to be the first tissue-specific mapping of NRG3 class expression in an extensive range of human tissue types, demonstrating that disease-related and genetically associated NRG3 classes II and III are brain-specific. Together, our results provide evidence for differential molecular organization of NRG3 splice isoform expression in human brain development, potentially relevant to the timing of specific neurodevelopmental events, including neuronal migration, cortical patterning, and neural circuit formation. The results also highlight particular splice isoforms in developmental patterns of risk and demonstrate that dysregulation of NRG3 expression occurs in several common psychiatric disorders, further implicating the NRG3 signaling pathway in broader psychiatric risk.
Discussion
Structural and polymorphic variation in NRG3 is associated with schizophrenia, bipolar disorder, and several neurodevelopmental disorders characterized by cognitive dysfunction (
1–
11,
20), implicating NRG3 as a potential key mediator of human neocortical development. We have previously demonstrated that NRG3 undergoes complex differential splicing in human neocortex and that rs10748842, a schizophrenia-risk-associated SNP, is a putative functional polymorphism associated with elevated NRG3 class expression in the brain (
7) and altered prefrontal cortical function (
12), highlighting potential molecular and neurobiological mechanisms of risk. We now find that NRG3 isoforms, including those modulated by risk-associated genomic variation, exhibit distinct expression trajectories during development and reveal alterations in balanced expression in the DLPFC of patients with mood disorders, as well as schizophrenia (
7). This new information provides insights into periods of potential sensitive vulnerability in the immature brain whereby dysregulation of NRG3 expression may contribute to the developmental origins of psychopathology, highlights potentially distinct biological roles for the different NRG3 isoforms, and suggests involvement of NRG3 in the shared biology of three common psychiatric disorders.
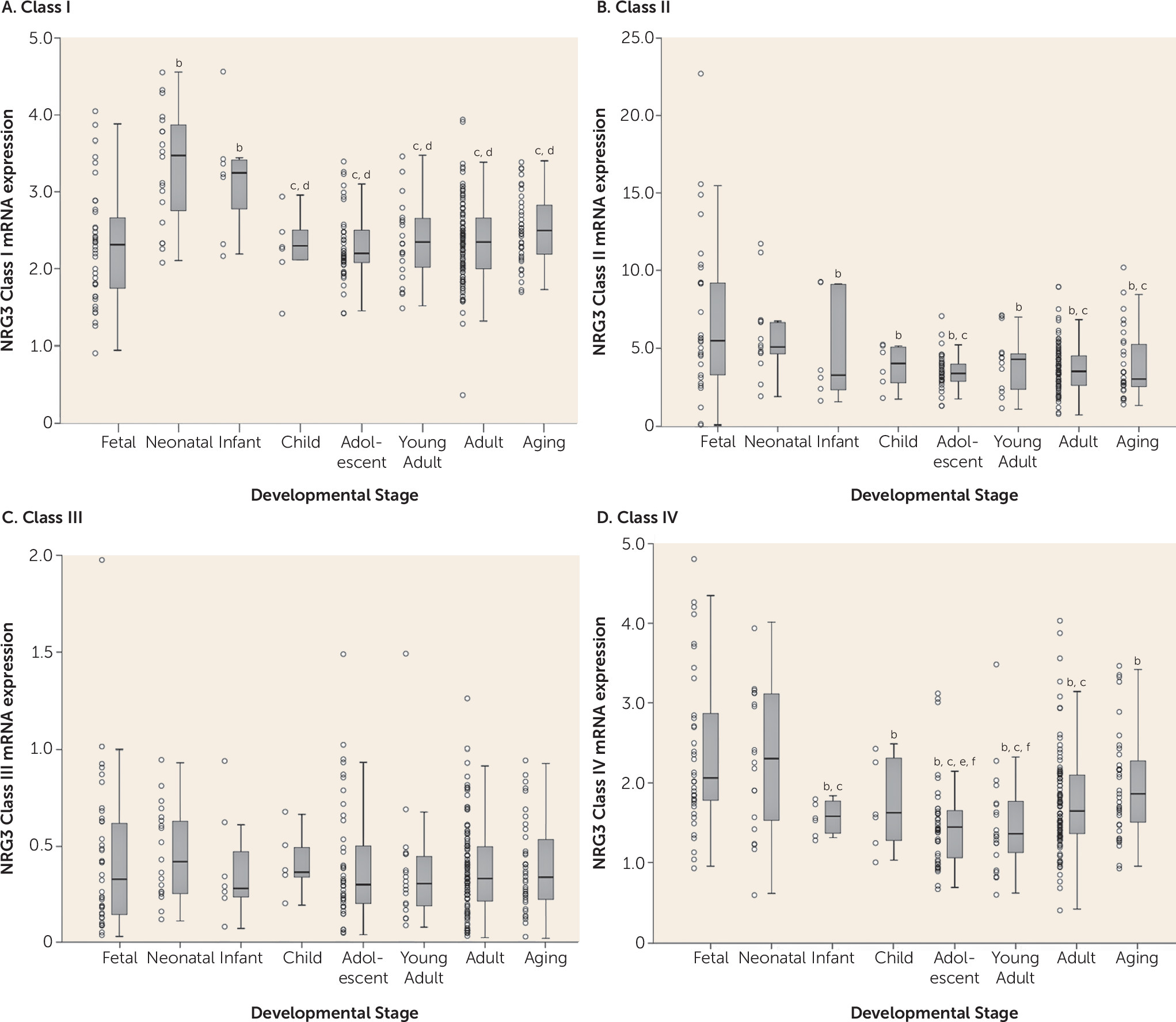
During brain development, gene expression patterns are highly dynamic and correlate with the growth and maturation of specific neuroanatomical systems (
31–
34), and recent molecular genetic studies suggest that variations in genes influencing cortical neurogenesis are involved in the pathogenesis of schizophrenia and related psychiatric disorders (
20,
21,
23,
35,
36). Our results provide quantitative molecular evidence that NRG3 isoform classes display unique neuro-ontogenic expression profiles in the human prefrontal cortex, potentially indicative of their key biological roles. As such, we show that NRG3 classes II and IV are dominantly expressed during the prenatal (second trimester) and neonatal period, decreasing to reach adult levels of expression by 1 year of age. Notably, the second trimester of brain development is characterized by major periods of neocortical neuronal migration, organization of the laminar structure of the cortex, and the development of thalamocortical projections (
32,
37–
39), while the neonatal period is exemplified by the rapid onset of synaptogenesis and oligodendrogenesis (
34). These findings emphasize a novel prospective role for NRG3 classes II and IV in human fetal and early postnatal prefrontal cortex development, perhaps consistent with murine studies showing NRG3′s critical contribution to cortical patterning and neuronal migration in the developing cortex (
14) and NRG3 class II proteins (hFBNRG3) as oligodendrocyte survival factors (
13). Interestingly, several lines of evidence support the centrality of the prefrontal cortex and altered executive function in schizophrenia and bipolar disorder (
40–
43); therefore it is particularly noteworthy that NRG3 class IV expression is increased in the DLPFC of patients with schizophrenia (
7) and class II is increased in bipolar disorder. These findings highlight a potential “immature” pattern of neocortical NRG3 isoform expression in schizophrenia (
7) and bipolar disorder and signify a potential developmental and molecular mechanism of risk contributing to altered prefrontal-mediated cognitive function (
8,
12).
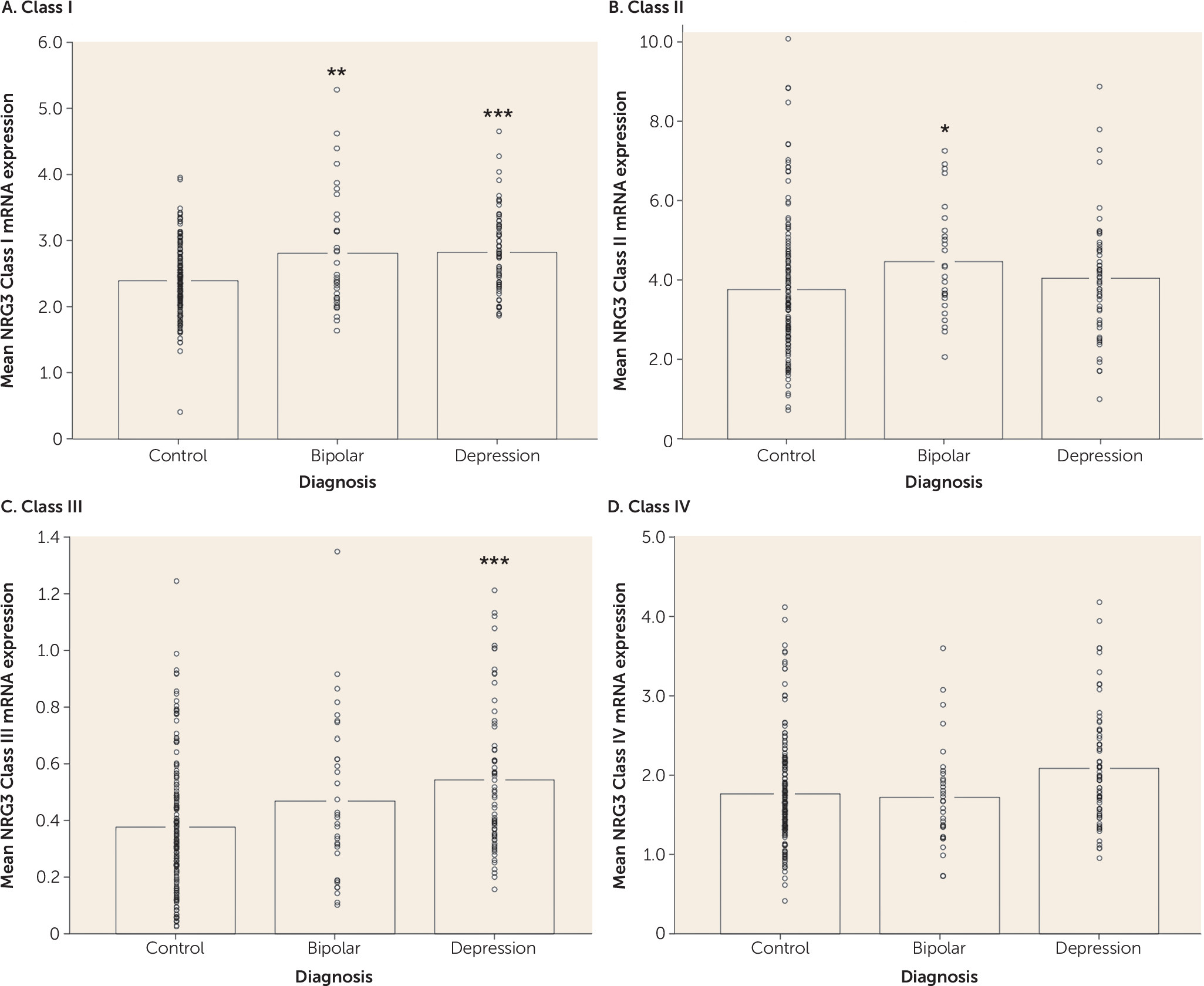
Consistent with a proposed biological role of NRG3 in human corticogenesis (
20,
44) and the suggested developmental origins of psychiatric illness (
45,
46), we also present data showing that NRG3 class I expression is significantly increased in bipolar disorder and depression, similar to findings in schizophrenia (
7). Interestingly, NRG3 class I isoforms display a unique developmental profile, being >1.5-fold lower in fetal brain, compared with peak expression at 0–3 years of age. This pattern suggests a key role for class I isoforms in postnatal brain development, a time of rapid cortical expansion, synaptogenesis, and progressive myelination (
34,
47), and demonstrates that all three common psychiatric disorders share a related molecular perturbation in NRG3. Nevertheless, how this relates to genetic risk is unclear. Although there is evidence of genetic overlap between severe psychiatric disorders, particularly schizophrenia and bipolar disorder, including NRG3 (
20,
21), the absence of association of rs10748842 and rs6548440 with class I expression levels suggests a state-related phenomenon. At present it is unclear whether changes in NRG3 class I are epiphenomenal or secondary to changes in other NRG3 isoforms.
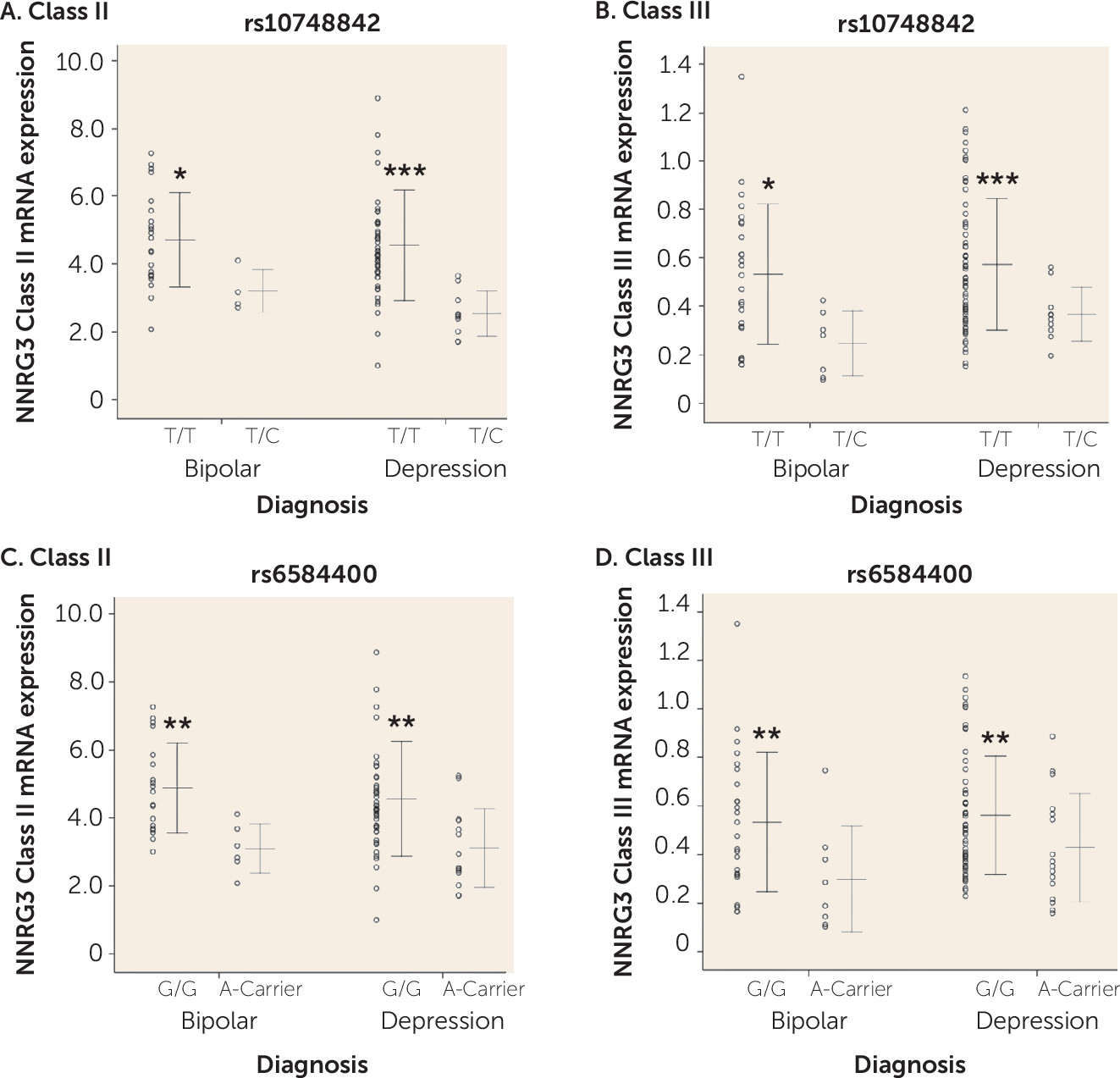
Polymorphisms in a 13-kb interval of NRG3 (rs10883866, rs10748842, and rs6584400) are associated with schizophrenia and several core phenotypic features of the illness (
7–
9,
12). NRG3 rs10748842 resides in a noncoding DNA ultraconserved element and is predicted to be central to a core transcription factor binding sequence for several key developmental transcription factors, including PAX and Hox (
7). Biologically, rs10748842 (T allele) is associated with increased expression of NRG3 classes II and III in the DLPFC of control individuals and patients with schizophrenia (
7). Our current data provide independent replication of this molecular association in patients with bipolar disorder and depression. Furthermore, we also identify subtle, but significant increases in NRG3 classes II and III in bipolar disorder and depression, respectively, in the same direction as the effects of the risk (T) allele, highlighting a potential molecular etiology underlying genetic risk at NRG3 for affective psychiatric illness and potentially consistent with recent GWAS data identifying NRG3 SNPs within intron 1 (including rs6584400) in the top pathways associated with bipolar disorder (
20,
44). As noted above, the developmental pattern of expression of these isoforms is distinct, with class II showing dominant expression in the fetal and neonatal cortex, perhaps consistent with a developmental component to this molecular genetic association, and class III showing maintenance of expression across all developmental stages, suggesting that the genetic association of rs10748842 genotype with class III expression is temporally agnostic. It is also worth noting that class III isoforms represent a potentially nontraditional NRG3 protein due to a truncated epidermal growth factor–like domain (
7). Analogous variants with unique signaling properties and cellular distribution have also been identified in the NRG1 family and shown to be relevant to risk for schizophrenia (
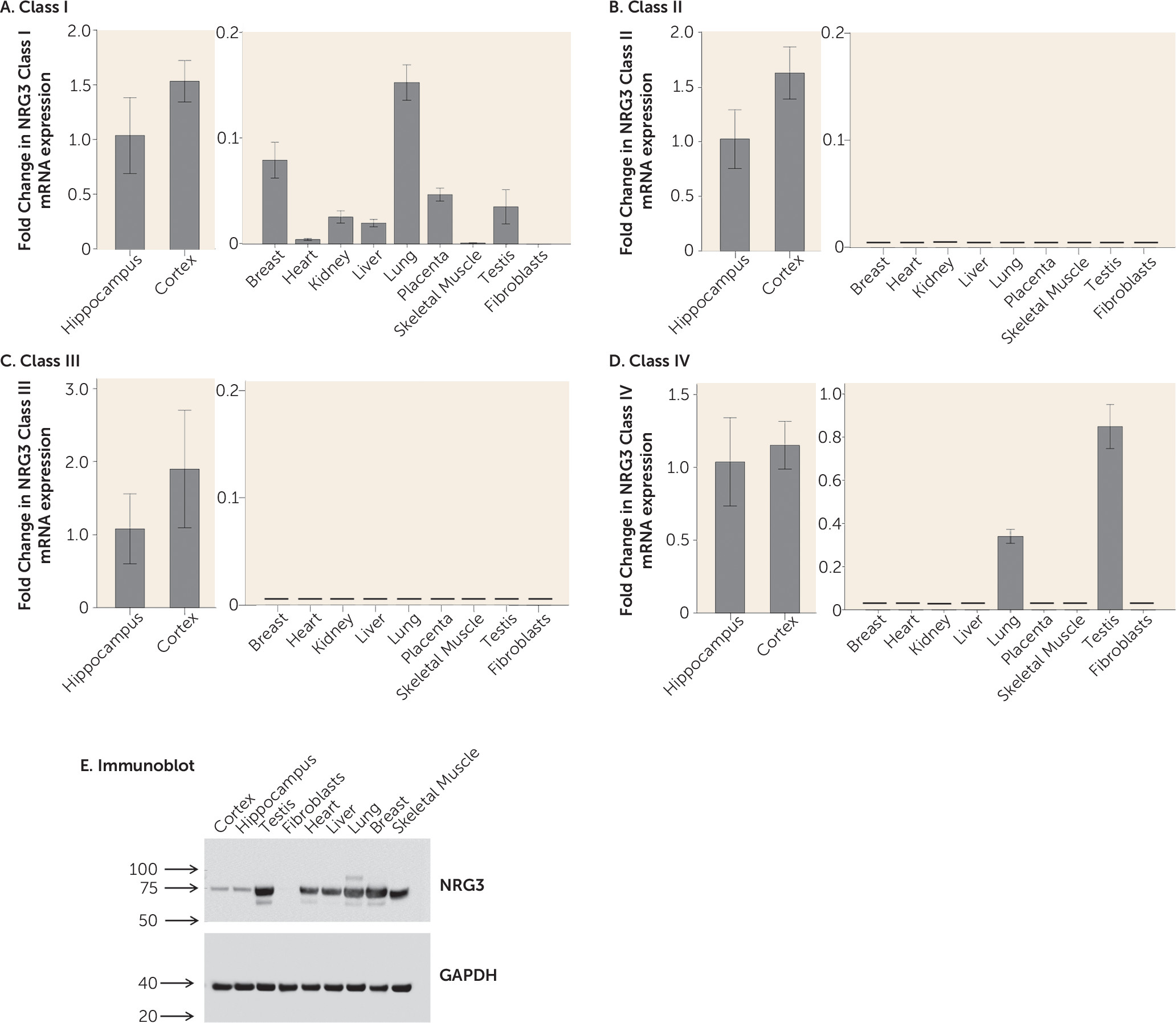
27). Moreover, tissue expression profiling strongly suggests that NRG3 classes II and III are brain-specific isoforms and we further identify that class III NRG3 expression is significantly associated with smoking status in the human brain. This is particularly interesting given NRG3′s recent genetic association with smoking cessation and nicotine dependence (
17,
48) and the increased incidence of nicotine dependence in psychiatric illness.
Finally, while NRG3 expression changes are relatively isoform- and disorder-specific, the directionality of expression changes is consistent with the observation that increased NRG3 signaling is pathologically relevant to psychiatric illness. NRG3′s receptor ErbB4 is also increased in a transcript-dependent manner in schizophrenia (
28,
49–
51). It is relevant that our recent development of a mouse model of NRG3 overexpression demonstrated that increased NRG3 signaling leads to sustained neurobehavioral deficits in adulthood (
16) and inhibition of neuregulin-ErbB4 mediated signaling has shown preclinical utility as a procognitive therapy (
52,
53).
In summary, our study provides an important framework for the mechanistic understanding of NRG3’s role in the developmental origins of neuropsychiatric illness and provides novel evidence for NRG3 expression regulation during critical periods of human pre- and postnatal brain development. The findings demonstrate that the NRG3 classes are nonredundant in their temporal, genetic, and tissue-type expression profiles and their involvement in psychiatric disorders and highlight the NRG3 signaling pathway as a potential target for psychiatric drug development.