Despite accumulated reports of a protective effect for lithium (
7–
9), no consistent evidence indicates whether it is superior to valproate in reducing suicide risk (
10–
14). Three meta-analytic reviews from Baldessarini’s group suggested significantly lower risks of attempted and completed suicides among lithium-treated patients than in either patients not treated with lithium (
8,
15) or patients treated with anticonvulsants (including valproate) (
16). A recent meta-analysis of randomized controlled trials for patients with mood disorders (
17) showed that lithium is more effective than placebo in reducing the number of completed suicides but was inconclusive about its ability to reduce the risk of suicide attempts more than either placebo or valproate. A landmark long-term randomized controlled trial was conducted among high-risk patients with bipolar disorder, and it detected no significant difference in the rate of suicide attempts between lithium and valproate, but the study was only powered to detect high relative risks (
18). More studies on valproate were conducted following an alert of increased risk of suicidal behavior related to anticonvulsants reported by the U.S. Food and Drug Administration, but the results gathered were contradictory (
4).
Observational studies avoid the ethical and logical problems encountered by randomized controlled trials and, additionally, have the advantage of large sample size with long-term follow-up, offering adequate numbers of rare suicide-related events. Nevertheless, observational pharmacoepidemiological studies are highly susceptible to confounding by indication, that is, patients are selected for a medication based on their risk for the outcome. For example, in Sweden, patients at high risk for suicide might be more likely to be given prescriptions for lithium because of previous reports of its antisuicidal properties. Valproate is believed to be more effective in rapid cycling and mixed states (
20), which can steer valproate prescriptions to these patients. Many approaches, including comparison of patients with different numbers of medication purchases (
7,
11,
12) and propensity score models accounting for exposure-related covariates (
14), are applied to address confounding, but these approaches cannot account for potential unmeasured confounding.
Method
Subjects
We linked longitudinal Swedish population-based registers, enabled by unique personal identification numbers (
22). By linking the Total Population Register, Migration Register, Cause of Death Register, National Patient Register, and Prescribed Drug Register (
23), we identified 51,535 individuals with bipolar disorder followed from Oct. 1, 2005, or age 15, or date of first diagnosis if later than Oct. 1, 2005, until emigration, death, or Dec. 31, 2013, whichever occurred first. This study was approved by the Ethics Committee at Karolinska Institutet.
Measures
Bipolar disorder.
We applied a modified validated algorithm (
24), which defined bipolar disorder as at least two inpatient or outpatient visits for a core discharge diagnosis of bipolar disorder (for International Classification of Disease [ICD] codes see Table S1 in the
data supplement accompanying the online version of this article). This algorithm includes bipolar I disorder, bipolar II disorder, bipolar disorder not otherwise specified, and schizoaffective disorder bipolar type and is sufficiently sensitive and specific to be used in Swedish register-based studies (
24).
Medications.
The main exposure was defined as medication with lithium sulfate (Anatomical Therapeutic Chemical [ATC] classification code: N05AN01) or sodium valproate/valproic acid (ATC code: N03AG01) in the Prescribed Drug Register. In routine psychiatric practice in Sweden, oral medications are unlikely to be dispensed for longer than 3 months at a time. Therefore, as was done in previous studies (
21,
25), we defined a medication period as a sequence of at least two prescriptions, with no more than 3 months (92 days) between any two consecutive prescriptions. Thus, for both lithium and valproate, individuals were defined as on medication during the time interval between two dispensed prescriptions, unless the dispensed prescriptions occurred more than 3 months apart. To determine whether an individual was on or off medication initially, the follow-up start was set to Oct. 1, 2005, because the coverage of the Prescribed Drug Register started on July 1, 2005.
Outcomes.
The main outcome was suicide-related events, defined as attempted or completed suicide (ICD-10: X60–X84, Y10–Y34), which included those with undetermined intent. Dates and diagnoses were retrieved from the National Patient Register and Cause of Death Register.
Statistical Analyses
To control for time-invariant covariates, we performed within-individual analyses using stratified Cox regression. This method, by design, exclusively draws information from individuals who ever attempted suicide during follow-up (i.e., individuals without suicide-related events or varying covariates during follow-up were noninformative). More methodological details are given in a related thorough review (
26).
We split the follow-up time into consecutive periods. A new period started after a medication switch (i.e., from off medication to on medication or vice versa, for either lithium or valproate) or a suicide attempt. For the latter, we restarted the following period at baseline (i.e., to set the underlying time scale as the time since the last suicide attempt). Lithium and valproate treatments were defined as time-varying dichotomous exposures, respectively. Age range (15–25, 26–38, 39–50, 51–100 years, grouped by the quartiles of the baseline age distribution among those who attempted suicide during the follow-up) and previous number of suicide attempts were adjusted for as time-varying categorical covariates. We estimated the hazard ratios and 95% confidence intervals (CIs) for differences in the rate of suicide-related events between periods. This method can be found in detail in related publications (
21,
25,
27,
28).
For significant associations between medication and suicide-related events, we estimated the population attributable fraction to assess the public health impact for patients with bipolar disorder. It measures the proportion of events that would be eliminated if the whole cohort would be medicated during the entire follow-up. The approaches for estimation and interpretation of population attributable fraction are described in the
online data supplement and elsewhere (
29). The CIs were estimated by nonparametric bootstrap methods.
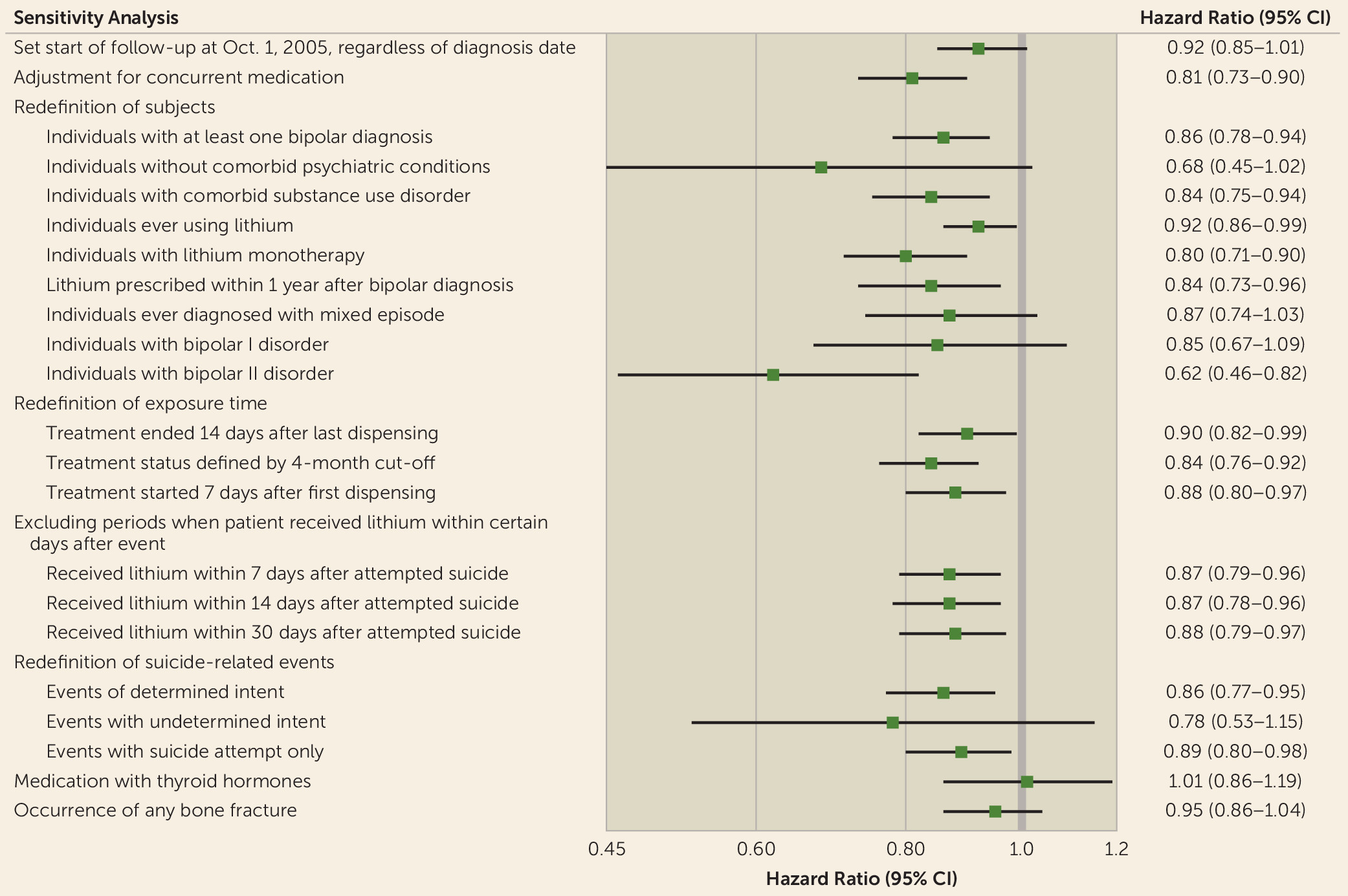
Sensitivity Analyses
We also performed standard between-individual Cox regression for comparison. Period splitting and covariate adjustment were the same as for the stratified Cox regression, with additional adjustments for sex, baseline severity of illness (measured by previous hospitalizations), and baseline history of suicide attempts. We estimated hazard ratios and 95% CIs with cluster-robust standard errors accounting for within-individual correlations. Furthermore, we used a different method, a propensity score model, for comparison. Since this method is commonly used for time-invariant exposures, we studied a shorter period without switch of medication status (the methods are described in the online data supplement).
For the significant association between lithium and suicide-related events, we conducted additional within-individual analyses to examine to what extent the association was affected by follow-up, inclusion criteria, and definitions of exposure and outcome.
First, we set the start of follow-up to October 2005 regardless of the date of bipolar disorder diagnosis. This was to take into consideration the possibility that early symptoms of adverse outcomes of illness might occur before diagnosis.
To examine confounding by other concomitant psychotropic treatment, we adjusted for concurrent medications, including lamotrigine, antipsychotics, antidepressants, benzodiazepines, and other anticonvulsants. The definitions of medication periods were the same as for lithium and valproate (for ATC codes, see the online data supplement).
To test for confounding by disease misclassification and comorbid conditions, we evaluated different inclusion and exclusion criteria. Instead of two admissions for bipolar disorder we 1) allowed for only one, 2) restricted analyses to patients with bipolar disorder without lifetime diagnoses of comorbid psychiatric conditions (for ICD codes, see Table S1 in the online data supplement), and 3) used all individuals to whom lithium was ever dispensed irrespective of diagnosis.
To better guide clinicians, additional research on the time and symptoms for which lithium versus another mood stabilizer should be employed as an antisuicidal treatment intervention is recommended (
19,
30). Therefore, we 1) conducted an analysis by restricting the cohort to individuals who were given prescriptions for lithium within 1 year after first diagnosis of bipolar disorder, to address the clinical question of whether lithium shortly after diagnosis is beneficial in preventing suicidal behavior, 2) analyzed a cohort of individuals whose records indicated they ever had a mixed episode, to investigate lithium’s effect for patients with potential high risks, and 3) performed analyses on two subcohorts of individuals with bipolar I and bipolar II disorder identified from the Swedish Bipolar Quality Register.
To assess potential misclassification of exposure periods or delayed onset of drug action, we 1) defined the end of each medication period as 14 days after the last dispensing date, 2) defined the medication period as a sequence of dispensed prescriptions with less than 4 months between them, and 3) set the start of a medicated period to 7 days after the first dispensing date.
To examine the robustness of our definition of suicide-related events, we repeated our analyses for events with 1) determined intent, 2) undetermined intent, and 3) exclusion of completed suicides.
To test whether the association was biased due to the assumption that a patient may be given a lithium prescription after attempting suicide, we repeated our main analysis excluding periods containing a switch to lithium medication within 7, 14, and 30 days after a suicide attempt, respectively.
It has been suggested that combination therapy with lithium plus valproate is more effective in preventing relapse than valproate alone (
31). To test the potential benefit of combination therapy for antisuicidal effect, we repeated our analysis by defining medication periods with lithium alone, valproate alone, and lithium plus valproate. Moreover, considering that patients with lithium monotherapy might be different from patients who have switched between lithium and valproate, we repeated our analysis for the subgroup with lithium monotherapy.
A recent study showed an increased risk of suicide after discontinuation of lithium (versus valproate) (
32). We also explored the risks after initiation or discontinuation of medication by further examining the time-varying effect (periods were categorized as on medication for less than 30 days/more than 30 days and off medication for less than 30 days/more than 30 days).
Finally, we used two negative controls to evaluate potential confounding. First, to test whether the association could be due to decreased likelihood of suicide during periods of active treatment, we examined risks of suicidal-related events during use of thyroid medications. Second, to test whether the inverse association is due to individuals receiving lithium prescriptions during less chaotic phases of illness, we examined the rate of any bone fracture during lithium medication.
All analyses were performed with Stata 13.0 (
33).
Results
Among the 51,535 patients with bipolar disorder, a total of 10,648 suicide-related events occurred in 4,643 individuals (9.0%) during 273,140 person years of follow-up (
Table 1). Lithium treatment was most prevalent (41.0%), followed by valproate (16.3%). About 50% of the patients were never exposed to lithium or valproate during the study period. The low percentage of patients on medications may partially be because a considerable proportion of patients identified from the register were older and had stopped taking medication. Between-individual analyses were performed among all the patients with bipolar disorder.
After excluding individuals without suicide-related events or varying covariates during follow-up, 4,405 individuals were eligible for the within-individual analysis. These patients had 10,403 suicide-related events, were more often prescribed other drugs, and had more comorbid psychiatric conditions.
We found a 14% reduced rate of suicide-related events for periods on compared with off lithium treatment (hazard ratio 0.86, 95% CI 0.78–0.95;
Table 2), but this was not the situation for valproate (hazard ratio 1.02, 95% CI 0.89–1.15). The test for the difference in hazard ratios for suicide-related events between lithium and valproate had a χ
2 of 4.29 (p=0.038). As in the main analyses, the between-individual analyses showed a lower rate of suicide-related events during lithium medication but not during valproate medication (
Table 2), with a significant difference between them (p=0.001). Periods on lithium medication constituted 17.9% of the total person-time for all patients. Based on the exposure rate and hazard ratios, the population attributable fraction was estimated as 12% (95% CI 4%−20%), which suggests that 12% of the suicide-related events could have been avoided if the patients would have been treated with lithium during the entire follow-up. The results remained consistent when we followed a shorter period with time-invariant medication by using 1:1 propensity score matching (hazard ratio 0.45, 95% CI 0.29–0.68 for lithium monotherapy; hazard ratio 0.93, 95% CI 0.50–1.73 for valproate monotherapy; see Table S4 in the
online data supplement).
Results for additional analyses to test the robustness of the association between lithium treatment and reduced suicide-related events are shown in
Figure 1. None of these analyses showed any substantive difference from our main results. The inverse association between suicide-related events and lithium remained after adjustment for other concurrent psychiatric medication (for hazard ratios for other psychiatric medications, see Table S2 in the
online data supplement). Subgroup analyses also showed reduced rates, although not all significant, for patients with bipolar I disorder (hazard ratio 0.85, 95% CI 0.67–1.09), bipolar II disorder (hazard ratio 0.62, 95% CI 0.46–0.82), and mixed episodes (hazard ratio 0.87, 95% CI 0.74–1.03). Notably, suicide-related events were less common (980 events in 24,631 patients) when we restricted the subjects to patients without comorbid psychiatric conditions; further investigation showed that the majority of suicide-related events occurred among patients with comorbid substance use (7,976 events in 15,927 patients) and that lithium remained associated with reduced suicide-related events in this group (hazard ratio 0.84, 95% CI 0.75–0.94). In contrast to the results for lithium, there was no evidence of an association between suicide-related events and thyroid therapy among patients with bipolar disorder (hazard ratio 1.01, 95% CI 0.86–1.19).
Analyses for lithium plus valproate are presented in Table S3 in the
data supplement and yielded no substantial difference from lithium alone. Results of medication periods defined by time-varying cutoffs are shown in
Table 3. It is noteworthy that patients had an increased rate of suicidal behavior within 30 days of lithium discontinuation (hazard ratio 1.33, 95% CI 1.09–1.61).
Discussion
Using a long follow-up period and what we believe is the largest sample ever reported, we found that rates of suicide-related events were significantly decreased during lithium treatment but not valproate treatment, with a possible difference between them. Since the within-individual analyses drew information exclusively from people who attempted suicide during follow-up, our results demonstrated that the association between lithium and reduced suicide-related events existed even among a high-risk population, which is unlikely to be studied in randomized controlled trials. Moreover, our suggestive between-drug differences supported evidence of unequal antisuicidal effects for lithium and valproate. Finally, we estimated that, in the absence of potential confounding, more than 10% of suicide-related events could have been prevented if all patients had been treated with lithium during the entire follow-up. By comparing treatment and nontreatment periods within the same individual, our approach automatically controlled for all time-stationary confounders and thus reduces the likelihood of confounding by indication in an observational study. Therefore, the observed inverse association supports the hypothesis of an antisuicidal effect of lithium.
Our finding of reduced suicide-related events during lithium treatment is in line with findings in many previous studies (
7,
8), including a recent U.K. study that found decreased rates of self-harm and unintentional injury after treatment with lithium and suggested that the mechanism could be a lithium-induced reduction in impulsivity (
14). It is worth noting that observational studies and meta-analyses constitute the main sources of evidence for lithium’s antisuicidal effects (
17,
19) and that adequately powered randomized controlled trials are lacking. Our results, with emphasis on high-risk patients, augment existing evidence and cumulatively support the hypothesis that lithium is protective against suicidal behavior. Additionally, our separate analyses on definite and uncertain events showed no material difference. Future research on the mechanisms behind the association between lithium and suicidal behavior is warranted and could inform the neurobiology of suicidal behavior.
In the analyses of people treated with lithium within 1 year after the first diagnosis of bipolar disorder, we observed similar reductions in suicide-related events, corroborating the positive effects of lithium prescription in this group (
34). Similar conclusions can be drawn for the high-risk group of those with mixed episodes. We observed nonsignificantly reduced suicide-related events during lithium treatment for bipolar I disorder and a significantly reduced rate for bipolar II disorder. This suggests that further exploration of subgroups within subtypes could be worthwhile. A history of substance use disorder predicts an increased rate of suicidal behavior in bipolar disorder (
3), and here we provide further evidence of lithium’s protective effect in this high-risk subgroup. Additionally, we observed a higher rate of suicide-related events shortly after lithium discontinuation. Although misclassification of medication periods cannot be ruled out, this finding, along with previous reports (
32), indicates a need for close monitoring after discontinuation.
For valproate, we found no protective effect for suicide-related events, with a significant difference between lithium and valproate. Moreover, the combination of lithium plus valproate showed no improvement. One study of U.S. Medicaid patients revealed a greater risk of suicide attempts among valproate users compared with lithium users, but no significant difference for completed suicide (
35). Two Danish studies found similarly reduced risks of completed suicide among consistent lithium and anticonvulsant purchasers, whereas for patients beginning anticonvulsant treatment, only a switch to or addition of lithium yielded reduced suicide rates (
11,
12). Compared with findings in the previous literature, our conclusion, by design, emphasizes the concomitant effect of drugs in patients with histories of medication switches and suicidal behavior. While current randomized controlled trials lack the power to detect a difference of effect between lithium and valproate (
18), our study suggests that there might be a distinct association between lithium and antisuicidal behavior, as compared with valproate (
36). This finding calls into question whether valproate can be said to have the same antisuicidal effect as lithium. It is therefore worrying that lithium use has decreased steadily during recent years in Sweden (
37), probably due to easier dose optimization and greater safety in case of overdose for valproate in clinical practice. It remains to be seen whether these changes alter the rates of suicidal behavior in the patient population.
Compared with clinical studies and randomized controlled trials, our large population-based longitudinal sample is representative of the population, thereby avoiding selective participation, which threatens validity and generalizability. The information on medication is complete and free from recall bias. To reduce selection effects and confounding by indication, we used a within-individual design that controls for unobservable time-invariant confounding for each person during the follow-up, as well as measured time-varying confounding.
However, within-individual designs cannot exclude the potential existence of unmeasured time-varying confounding within each person, including varying severity of symptoms of illness (e.g., rapid cycling) (
38), frequency of hospital visits, treatment site, and other types of concomitant treatment.
We tried to address many possible alternative explanations for the inverse association between lithium and suicide-related events. Consistently, no material differences were found in a series of sensitivity analyses (
Figure 1), indicating that misclassifications of disease, exposure, and outcome are unlikely to invalidate the results. To account for the effect on prescription by previous suicide history, our main models set the underlying time scale as the time since the last suicide attempt. Unavoidable limitations include the lack of information regarding adherence, similar to the situation in intention-to-treat analyses in randomized controlled trials, but if anything, this would result in underestimation of our reported associations. Our conservative way of defining the end of the medication period is another possible source of underestimation (i.e., individuals classified as off medication at the date of last dispensed prescription were probably truly medicated). Lithium’s antisuicidal impact might have a delayed effect after the beginning of treatment, but our sensitivity analysis using different measures of start, end, and length of medication period resulted in consistent estimates. One hypothesis posits that the reduced rate of suicidal behavior is due to active treatment rather than the effect of the drugs themselves. Another source of confounding could be that factors impacting a change in medication (e.g., stressful life events) may also impact the risk of suicidal behavior. To test for these hypotheses, we estimated rates of suicide-related events among patients who received thyroid hormone medication (which is believed to have no effect on bipolar disorder or suicidal behavior) and found no associations. Furthermore, we found no association between lithium treatment and the rate of bone fractures, which suggests that lithium’s effect could be suicide-specific. Finally, we tested the findings with follow-up of periods without medication changes and used propensity score analyses to limit potential confounding measurable at baseline; the conclusions remained consistent.
Despite these efforts, observational studies like ours cannot exclude a potential lithium-specific effect due to the required routine blood tests during lithium treatment to monitor for rare side effects. It is also possible that clinicians hesitate to prescribe lithium to high-risk patients because of the potential for lethal toxicity in case of overdose. Altogether, more evidence from randomized controlled trials with large samples and long follow-up are warranted to address these questions.
Three additional issues should be considered when interpreting the results. First, we cannot evaluate the associations with completed suicide directly, as stratified Cox regression is not applicable to nonrepeated events. However, the percentage of completed suicides was small (590 in 10,648 suicide-related events, 5.5%), and analyses excluding these events yielded similar results. Second, the estimate of population attributable fraction applies to our study population only during the follow-up period, since the estimation is based on exposure proportion and thus will vary depending on prescription rates and medication adherence. Third, it would have been ideal to have information on suicidal behavior for patients who had switched between lithium and valproate; however, we lacked power to perform this critical comparison.
In summary, we demonstrated an association between lithium and reduced suicide-related events, whereas the results provided no equivalent effect of valproate. Our results, in conjunction with existing literature, indicate that in patients with bipolar disorder and suspected suicidal intentions, lithium should be considered as a suicide preventive strategy, with a balance between efficacy and tolerability.