Complementary Features of Attention Bias Modification Therapy and Cognitive-Behavioral Therapy in Pediatric Anxiety Disorders
Abstract
Objective:
Method:
Results:
Conclusions:
Method
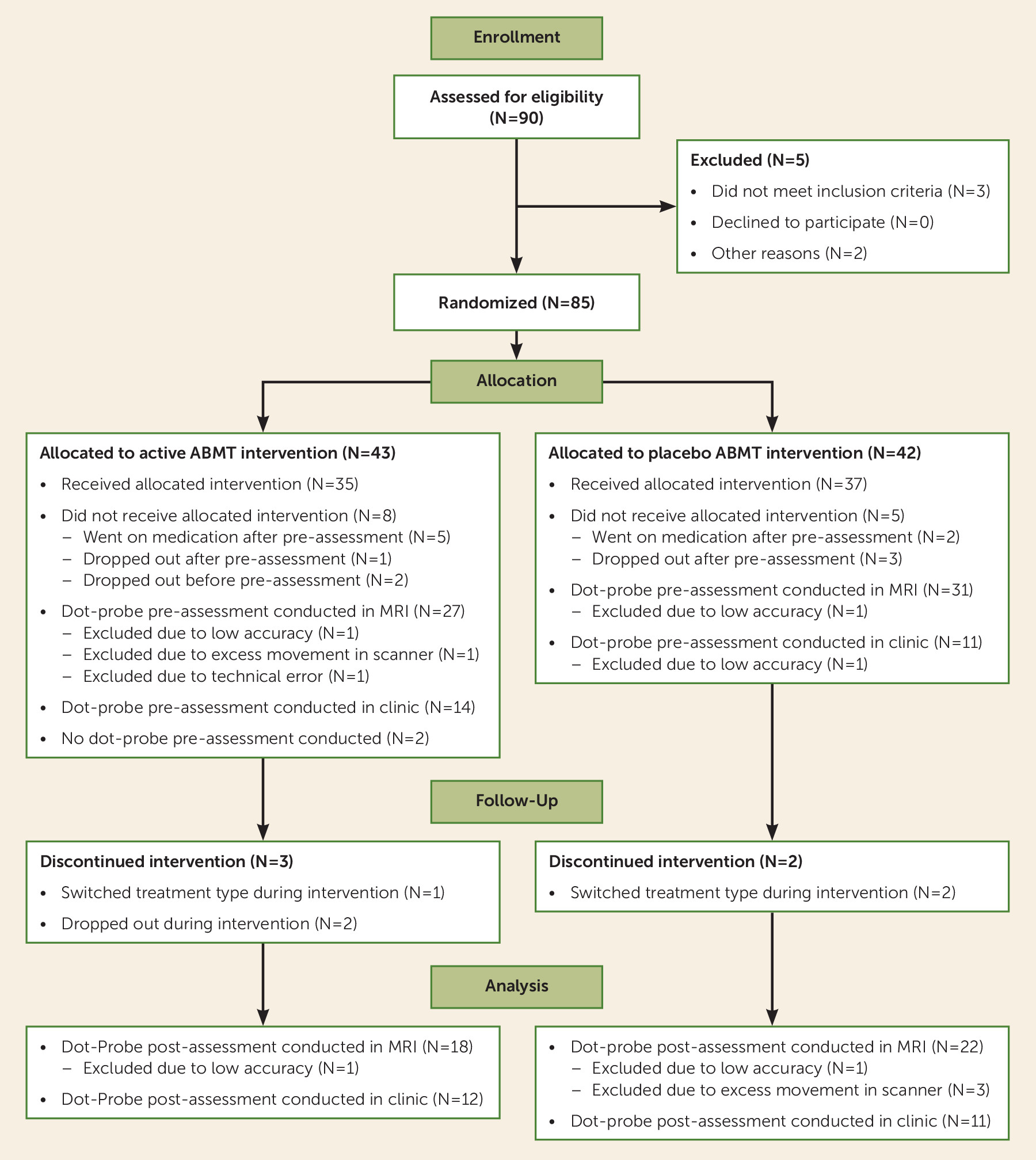
Participants
| Characteristic or Measure | Anxiety Group (N=54) | Healthy Comparison Group (N=51) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 32 | 59.3 | 23 | 45.1 |
| Mean | SD | Mean | SD | |
| Age (years) | 12.08 | 2.80 | 12.86 | 1.94 |
| IQ | 112.78 | 15.55 | 113.18 | 11.58 |
| Baseline SCARED total scorea | 29.40 | 9.59 | 5.44 | 4.68 |
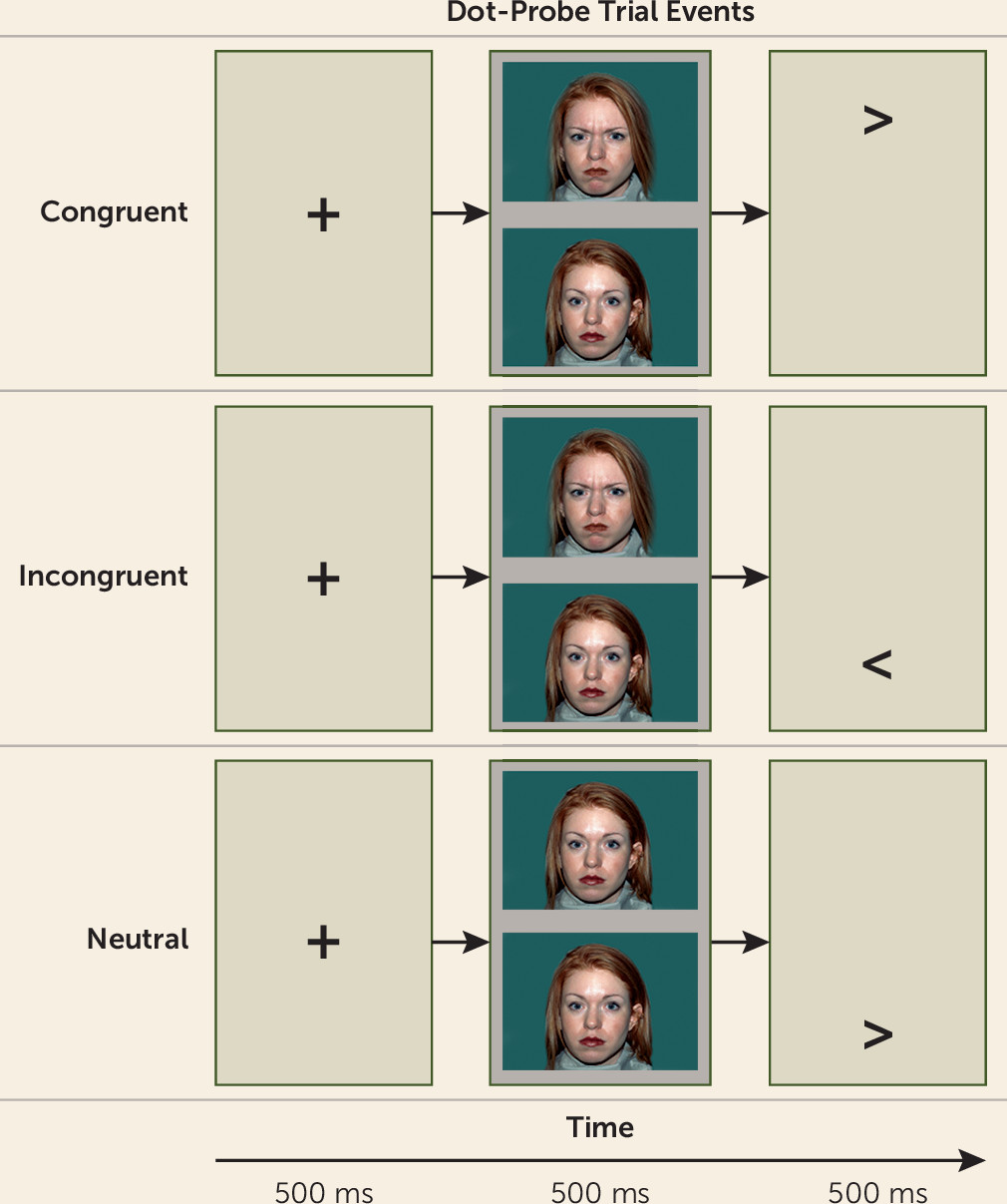
Attention Bias Assessment
Treatments
Clinical Treatment Data Analysis
Imaging Data Acquisition and Analysis
fMRI acquisition parameters.
fMRI preprocessing.
fMRI data analysis.
Pretreatment amygdala connectivity:
Amygdala connectivity and treatment response:
Results
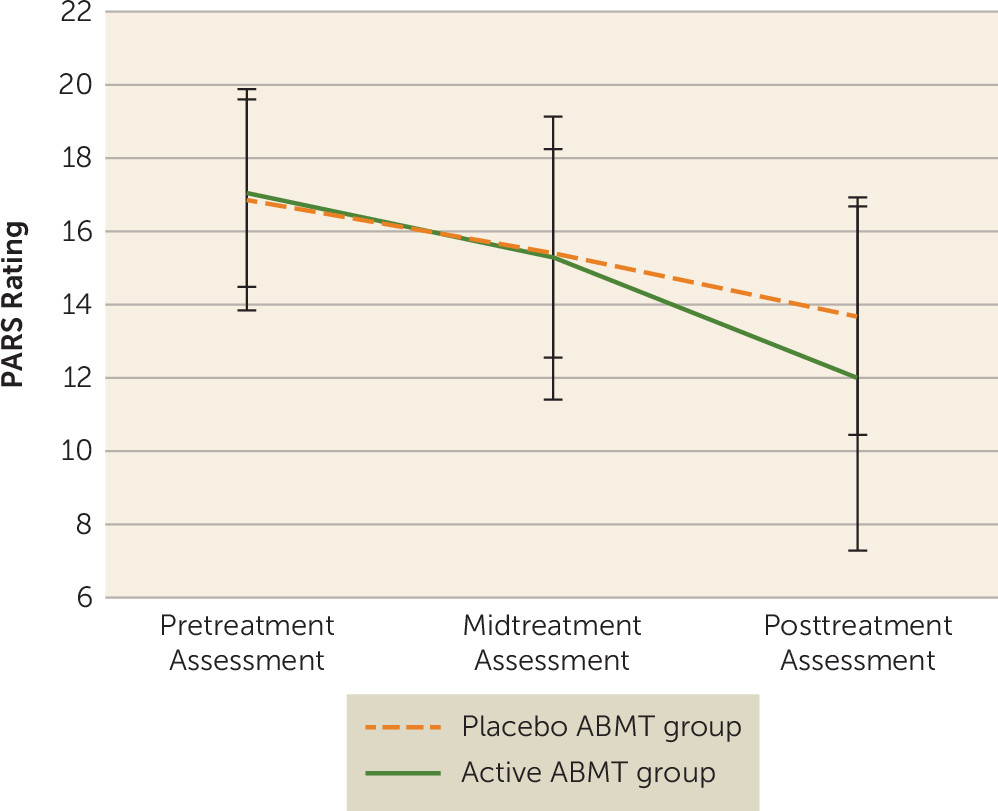
Clinical Effects of CBT and ABMT
| Characteristic or Measure | Active ABMT Group (N=43) | Placebo ABMT Group (N=42) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 26 | 60.5 | 24 | 57.1 |
| Mean | SD | Mean | SD | |
| Age (years) | 11.62 | 2.78 | 11.79 | 2.73 |
| IQ | 110.42 | 14.66 | 114.00 | 15.50 |
| PARS rating | ||||
| Pretreatment assessment | 17.03 | 2.56 | 16.84 | 3.03 |
| Midtreatment assessment | 15.26 | 3.86 | 15.38 | 2.85 |
| Posttreatment assessmentb | 11.97 | 4.69 | 13.67 | 3.25 |
| CGI-I | ||||
| Midtreatment assessment | 3.86 | 0.80 | 4.23 | 0.69 |
| Posttreatment assessment | 3.35 | 0.88 | 3.29 | 0.97 |
Pretreatment Amygdala Connectivity
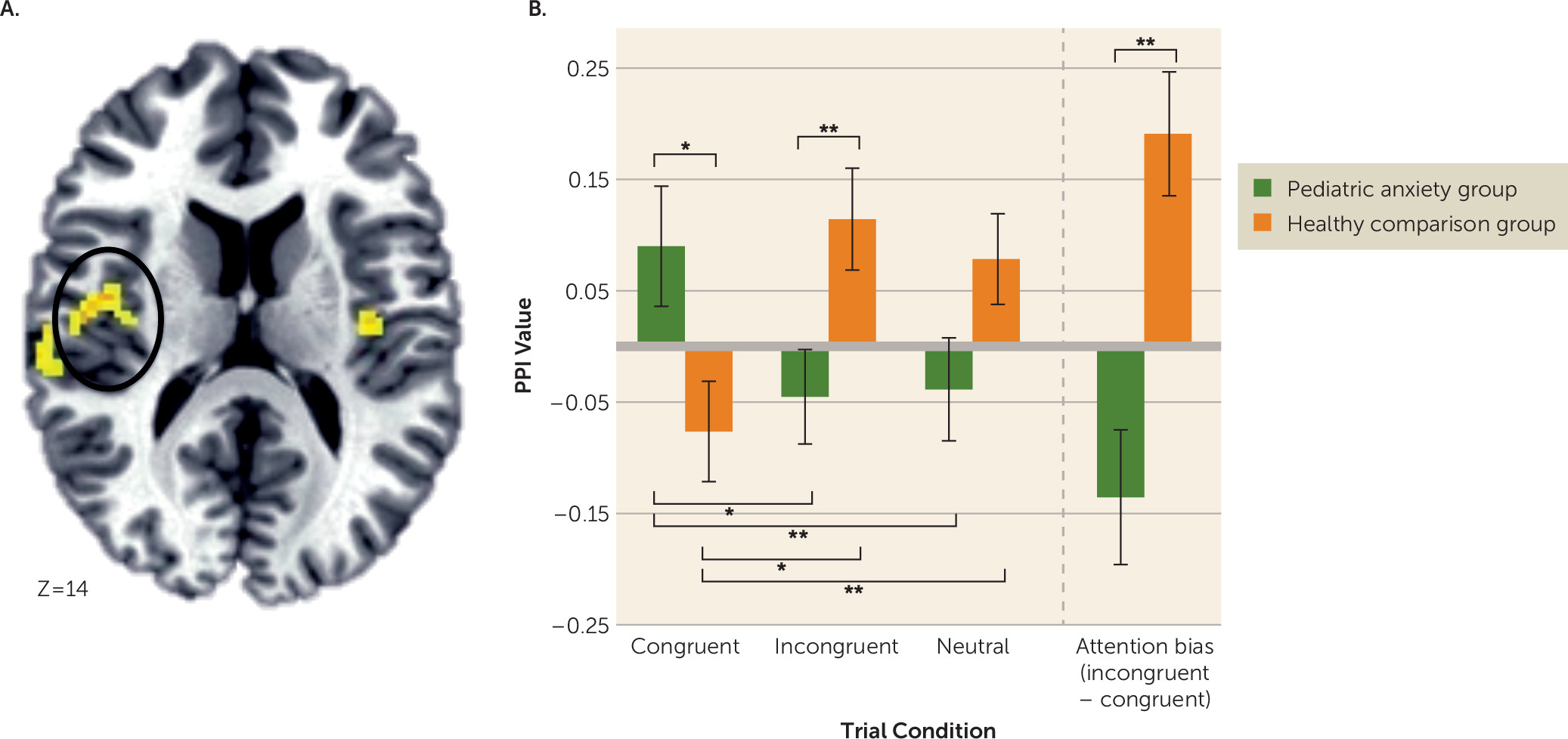
Amygdala Connectivity and Overall Treatment Response
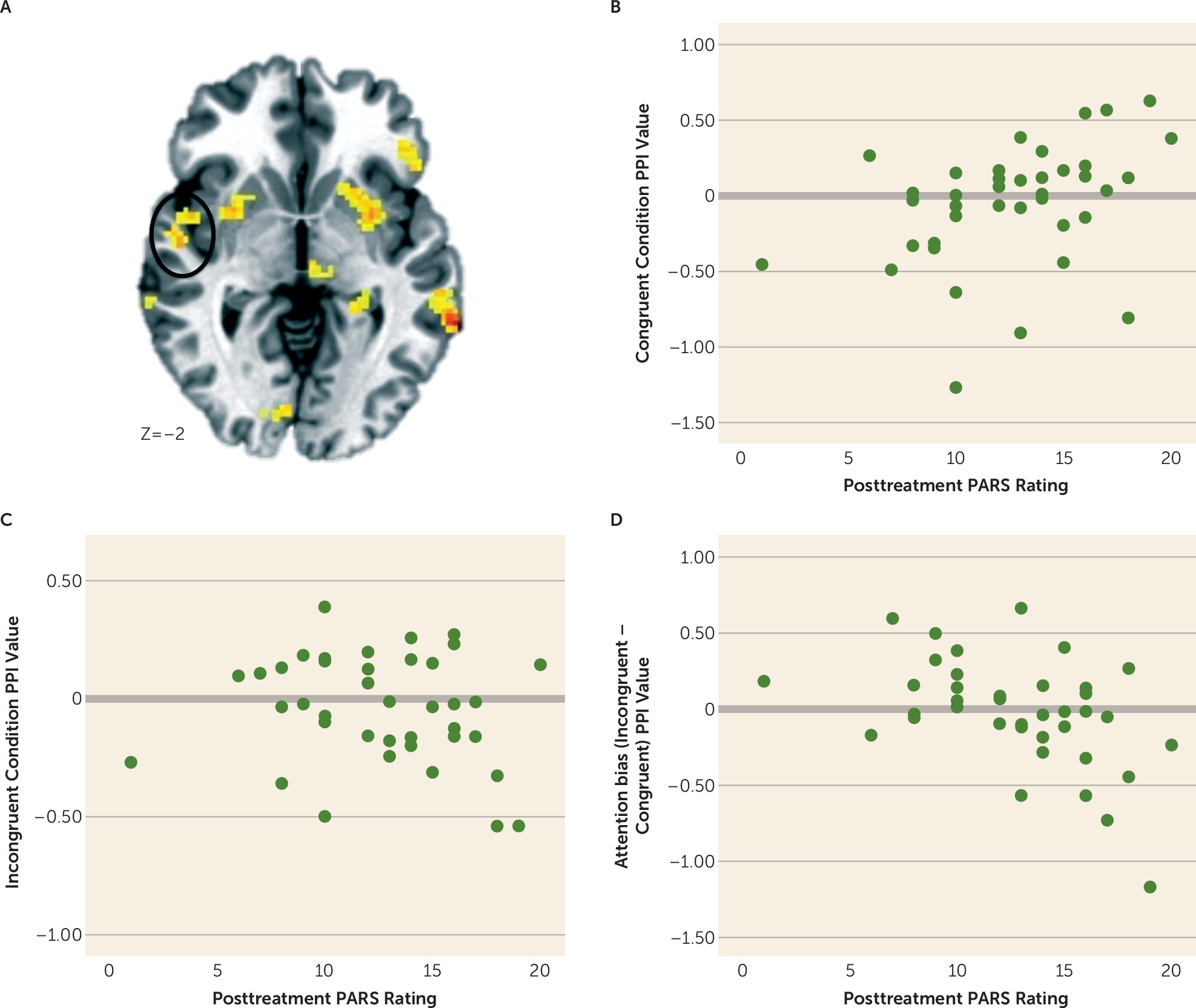
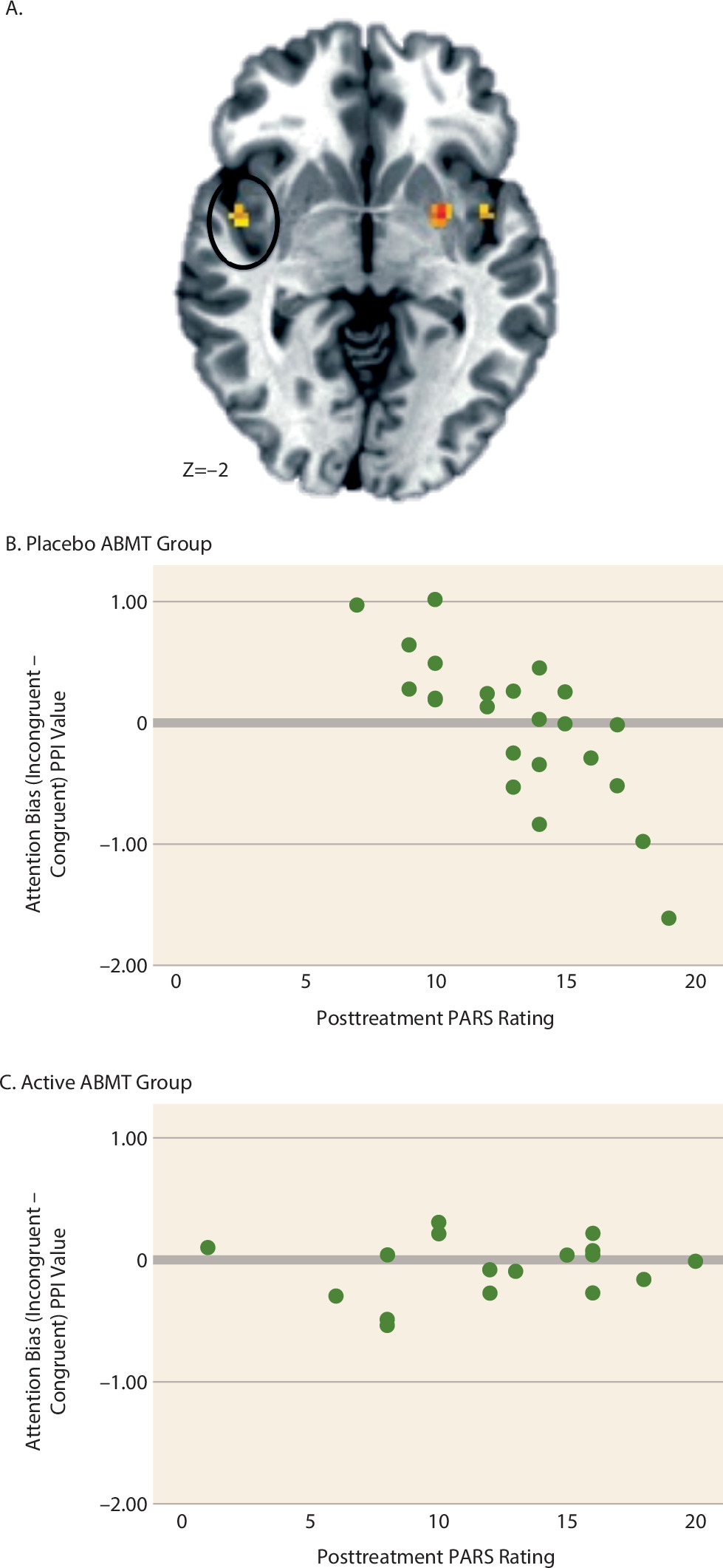
Amygdala Connectivity and ABMT-Specific Response
Discussion
ABMT Augmentation of Clinical Response
Amygdala-Based Functional Connectivity and Anxiety Disorders
Amygdala-Based Connectivity and Treatment Outcome
Limitations and Conclusions
Footnote
Supplementary Material
- View/Download
- 388.51 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.

