Exposure to early-life adversity disrupts brain development, resulting in altered brain networks and structure (
1–
3). These structural changes are associated with functional alterations that are often maintained throughout the lifespan, mediated by enduring epigenetic modifications of gene expression (
2–
4). Indeed, enduring alterations of brain structure and function at molecular, cellular, and circuit levels (
5,
6) are considered fundamental mechanisms of how early-life experiences influence health and disease (
1–
5). In the aggregate, exposures to intrauterine and neonatal insults (
7) and consequent altered brain anatomy (
8,
9) and connectivity (
10) contribute significantly to the global burden of mental illness.
Specifically, early-life exposure to maternal stress and trauma is linked to subsequent depression (
11–
13), posttraumatic stress disorder, panic disorder, substance abuse, abnormal stress response (
13,
14), and other serious disorders (
2,
3,
11). Emerging evidence suggests that exposure to early-life adversity may be causally related to brain changes underlying the risk for psychopathology (
7,
15,
16). Because the fetal period is unmatched by any other in growth and development, this stage in the human lifespan is the most vulnerable to both organizing and disrupting maternal signals.
Among the most salient signals shaping the human fetus is the stress hormone corticotropin-releasing hormone (CRH). CRH, a 41-amino-acid neuropeptide that is normally synthesized primarily in the paraventricular nucleus of the hypothalamus, has a major role in regulating pituitary-adrenal function and the physiological response to stress (
17,
18). CRH synthesized and released in other brain regions, such as the hippocampus (
19) and the cortex (
20,
21), contributes to the sculpting of neuronal dendritic development and maturation via its actions on specific receptors that are located on dendritic spines. Indeed, nanomolar concentrations of CRH have been shown to cause excessive pruning of dendritic trees of developing rodent cortical neurons (
22). CRH of brain origin is not detectable in the circulation (
23). However, during human pregnancy, the CRH gene, located on the long arm of chromosome 8 (
24), is expressed in the human placenta and amniotic membrane (
25,
26). Placental CRH (pCRH) is released into the maternal and fetal compartments as early as the eighth week of gestation and increases exponentially across gestation to regulate fetal maturation (
27), metabolic functions (
28,
29), and the timing of birth (
30).
pCRH expression is responsive to a range of maternal stress signals, including increased cortisol, norepinephrine, and epinephrine; reduced uterine blood flow; and infection (
31–
33). Thus, pCRH represents an integrative pathway through which diverse prenatal stressors inform the fetus of the state of its environment and shape fetal developmental trajectories in preparation for life after birth (
34,
35). There are several reports linking elevated human fetal exposure to pCRH, including links to decreased fetal startle and habituation (
27,
36); delayed neonatal neuromotor development (
37); increased infant fear and distress (
38); and prodromal markers of increased risk for affective disorders in young children (
39). Here, we demonstrate the potential consequences of fetal exposure to elevated levels of CRH and identify putative mechanisms. Specifically, we find that higher levels of pCRH are associated with cortical thinning in selective brain regions and with decreased cognitive and emotional function in preadolescents.
Method
All methods, human procedures, and protocols were approved by the Institutional Review Boards of the University of California, Irvine, the University of California, Los Angeles, and the Cedars-Sinai Medical Center, Los Angeles. Mothers gave written informed consent for all aspects of the protocol, and children (ages 6–9) provided assent.
Participants
Ninety-seven mother-child dyads participated in a longitudinal study of the effect of fetal exposure to maternal stress hormones on child MRI measures (see the CONSORT diagram in Figure S1 in the data supplement that accompanies the online edition of this article; see also Tables S1A–C in the data supplement). Women provided informed consent to provide blood samples at five intervals during gestation: 13.5–16.6 weeks (mean=15.3 weeks), 17.8–20.5 weeks (mean=19.2 weeks), 23.7–26.5 weeks (mean=24.9 weeks), 29.9–32.3 weeks (mean=30.9 weeks), and 34.6–38.1 weeks (mean=35.9 weeks). All women were English-speaking healthy adult (>18 years of age) pregnant women with singleton intrauterine pregnancies. Women were excluded if they had multiple births; used tobacco, alcohol, or others drugs during pregnancy; had uterine or cervical abnormalities; or had any conditions associated with dysregulated neuroendocrine function. Their children (48 boys, 49 girls) were enrolled in the study at 6–9 years of age (mean=7.3 years [SD=0.91]).
Assessments of pCRH in Pregnant Women
Gestational age at testing was determined by last menstrual period and was confirmed by obstetric ultrasonographic biometry before 20 weeks. Maternal blood samples (20 mL) were collected serially at five intervals throughout pregnancy by antecubital venipuncture into siliconized EDTA (purple top) Vacutainers and then immediately chilled to 60°C. Samples were centrifuged at 2,000 g for 15 minutes, decanted into polypropylene tubes prepared with aprotinin (Sigma Chemical, St. Louis; 500 KIU/mL blood), and stored at −80°C until assayed.
pCRH Determination
Following previously reported methods (
40), the concentration of total maternal pCRH was determined by radioimmunoassay (Bachem Peninsula Laboratories, San Carlos, Calif.). The CRH assay had less than 0.01% cross-reactivity with ovine CRH, 36% cross-reactivity with bovine CRH, and nondetectable reactivity with human ACTH. The intra- and interassay coefficients of variance ranged from 5% to 15%. The minimum detectable dose of the assay is 2.04 pg/mL (see the
online data supplement for additional assay details).
Data reduction for the radioimmunoassay was conducted with a computer-assisted four-parameter logistics program (
41). Values exhibiting greater than 25% error (deviation from the standard curve) were not included in the analyses. A subset of samples (N=60) was sent to a clinical laboratory (Quest Diagnostics) for further validation. The correlation between the two sets of data was 0.87 (p<0.01). Shared variance between 19- and 31-week CRH samples was a modest 14% (r=0.38). Mother-child dyads were recruited from two separate studies with identical prenatal assessments. To ensure comparability between the cohorts for analysis, the pCRH values were standardized within the two groups and then combined for statistical analysis (see the
data supplement for details). As expected, pCRH levels increased geometrically as gestation advanced (see Figure S4 and Table S9 in the
data supplement).
Assessments in Children
All children (mean age, 87.3 months [SD=10.9]) had a stable neonatal course (median Apgar score, 9 [range, 8–10]; gestational age at birth, 39.2 weeks [SD=1.5]) and were without known neonatal illness or congenital, chromosomal, or genetic anomalies. Participants had no evidence of neurological abnormalities in the newborn period. Children’s structural MR images were assessed for normal anatomical appearance. Seven children with motion artifacts and three with abnormal scans were not included in the final sample (N=97). These 10 children did not differ significantly on any measure from those whose scans were usable (see Table S8 in the
data supplement). At 6–9 years of age, no physical conditions were reported by the parents in a structured interview format of the MacArthur Health and Behavior Questionnaire (
42). The majority of children (88%) were right hand dominant (Edinburgh Handedness Inventory) (
43).
Structural MRI Acquisition
The structural MRI scan was acquired with a 3-T Philips Achieva system. Children received earplugs and watched a movie while in the scanner to increase compliance and minimize movement. A high-resolution T1 anatomical scan was acquired in the sagittal plane with 1 mm3 isotropic voxel dimensions. An inversion-recovery spoiled gradient recalled acquisition sequence with optimal parameters was applied: TR=11 ms, TE=3.3 ms, TI=1,100 ms, turbo field echo factor=192, number of slices: 150, no sensitivity encoding (SENSE) acceleration, flip angle=180°, shot interval (time from inversion pulse to the center of acquisition)=2,200 ms. The images were reviewed by the MRI operator (who was unaware of any of the study parameters) immediately after the scan was completed. If there were visible signs of motion artifacts, the subject was asked to stay for an additional scan. If the subject agreed, a second scan was acquired.
Processing of MRI Data
Cortical surface reconstruction and volumetric segmentation were performed with FreeSurfer (
http://surfer.nmr.mgh.harvard.edu/). Streamlined image processing procedures included application of intensity normalization prior to segmentation to minimize errors in identifying the boundaries (
44); removal of nonbrain tissues (
45); and transformation of images into Talairach space. Pial and white matter surfaces were located by finding the highest intensity gradient. Surface inflation was applied to each individual brain (
46), and the inflated brains were registered to a spherical atlas. Cortical thickness was the closest distance from the gray matter or white matter surface to the pial surface at each vertex on the tessellated surface (
47).
Assessment of Percentage of Cortical Areas Affected by Fetal Exposure to pCRH
Smoothing of images was done prior to regression. The command line interface was used for false discovery rate corrections and to accommodate more than one nuisance variable. Gestational age at birth, birth weight, age of the child at testing, sex, and handedness were included as covariates. A threshold of p<0.05 was used for all statistical tests, and outcomes were corrected for multiple comparisons using false discovery rate.
After false discovery rate corrections and spatial normalization, the number of vertices that were significantly associated with pCRH was determined in each region of the cortex. The numbers of significant vertices were summed for each region and divided by the total number of vertices in that area to provide the percentage of vertices that were significantly associated with pCRH concentrations. The same procedure was used for the number of significant vertices in each lobe. For hemispheric and whole brain percentages, the procedure was the same except that the total number of subcortical vertices was subtracted from the total.
Child Behavioral Analyses
We conducted a battery of tests that interrogate several brain regions and circuits to obtain a relatively broad assessment of cognitive and emotional function.
The Child Behavior Checklist (
48) is one of the most widely used parental interviews for identifying affective and conduct disorder problems in children. Structured interviews (rather than a questionnaire) were administered to mothers about their children’s behavior. We focused on two inclusive scales; one that assessed internalizing problems (sum of anxious-depressed, withdrawn-depressed, and somatic-complaints scores) and one that evaluated externalizing problems (sum of rule-breaking and aggressive behavior problem scores). Responses were recorded on a Likert-type scale ranging from 0 (not true) to 2 (very true or often true). Scores were summed, and standardized scores were computed that are age and sex specific.
Reaction Time to Incongruent Stimuli
The flanker task is an executive function task that requires the ability to resolve conflicts when competing information is present (
49). Participants view five arrows arrayed horizontally on a screen and are instructed to press a left or right response button based on the direction of the center arrow (target). They are instructed to ignore the surrounding arrows, which are either congruent (all aligned in the same direction) or incongruent with the center arrow. This task consists of 24 congruent trials and 24 incongruent trials. Each set of arrows is presented until the child responds (maximum of 5,000 ms), with a 750-ms intertrial interval. Among the scores, median reaction time to targets with incongruent distractors correlates most highly with other indexes of performance derived from this test (all r values >0.70). The association between externalizing scores on the Child Behavior Checklist and the median reaction time on the flanker task was not significant (r=0.09, p=0.41).
Results
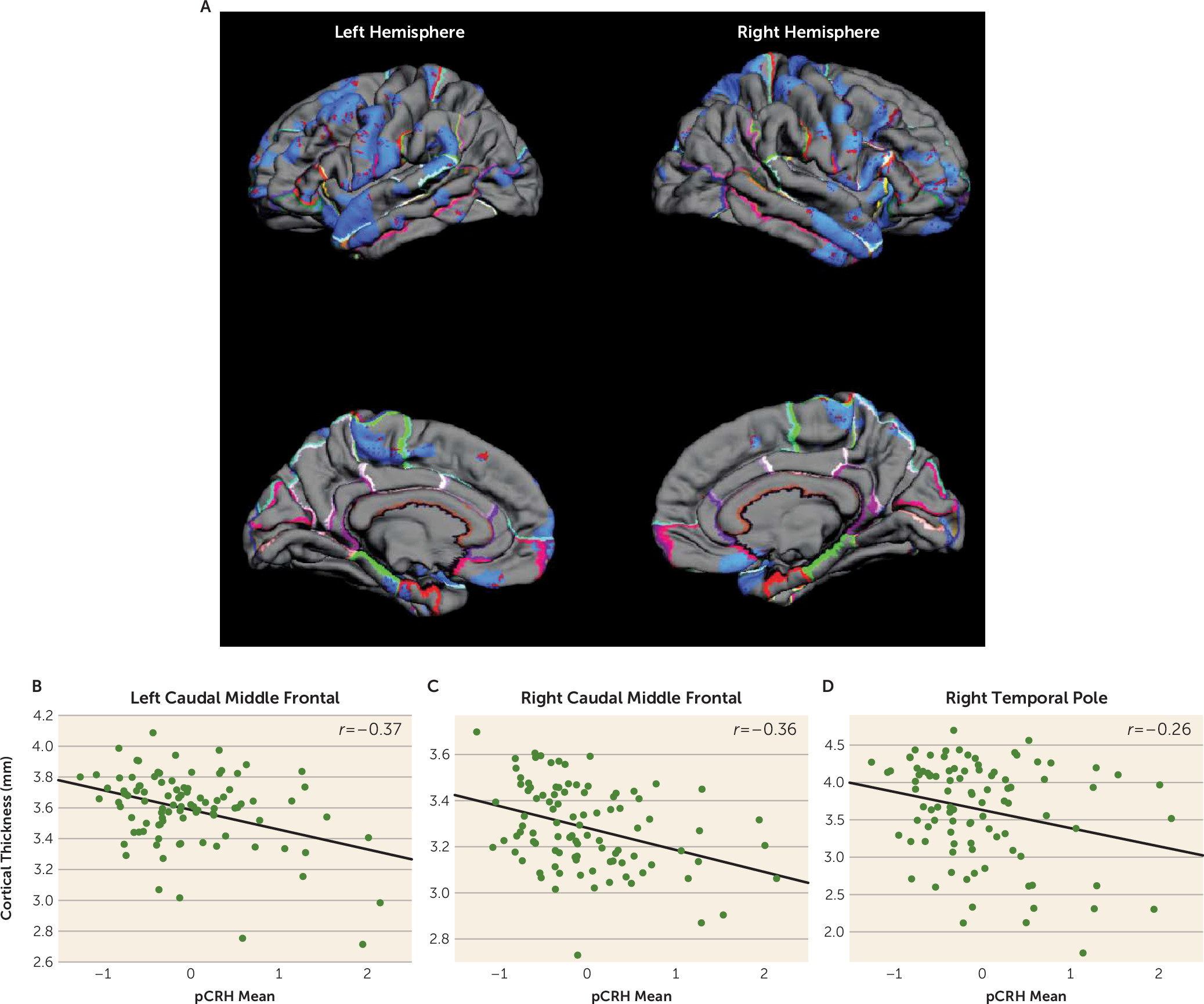
Elevated CRH Levels Throughout Gestation: Association With Cortical Thinning
Significant associations were observed between fetal exposure to pCRH averaged across gestation and areas of cortical thinning in children, as illustrated in the pial maps in
Figure 1. Children exposed to high levels of pCRH throughout fetal life exhibited significant thinning in 12% of the whole cortical mantle (see Table S2A in the
data supplement). The anatomical distribution of the cortical thinning associated with CRH exposure involved the left and right hemispheres equally (12% and 11% of hemisphere reduced, respectively) (see Table S2A in the
data supplement). Regional analyses indicated that cortical thinning associated with pCRH levels was primarily in the temporal (15%) and frontal (16%) regions (
Table 1). Panels B–D in
Figure 1 are illustrative scatterplots of the associations between total fetal exposure to pCRH across gestation and cortical thinning in the frontal and temporal areas (all significant, p<0.01–0.001). The representative scatterplots, coupled with the data in
Table 1, highlight the widespread association between fetal exposures to pCRH and regional cortical thinning, which is most notable in the temporal and frontal regions. Average concentrations of pCRH across gestation or levels at any gestational interval were not associated with increased cortical thickness either globally or in any region (see Table S2B in the
data supplement). Focusing on two gestational ages, 19 weeks (early, when pCRH production begins to accelerate) and 31 weeks (late, the time strongly associated with preterm birth), we found that maternal levels of pCRH were significantly associated with cortical thinning.
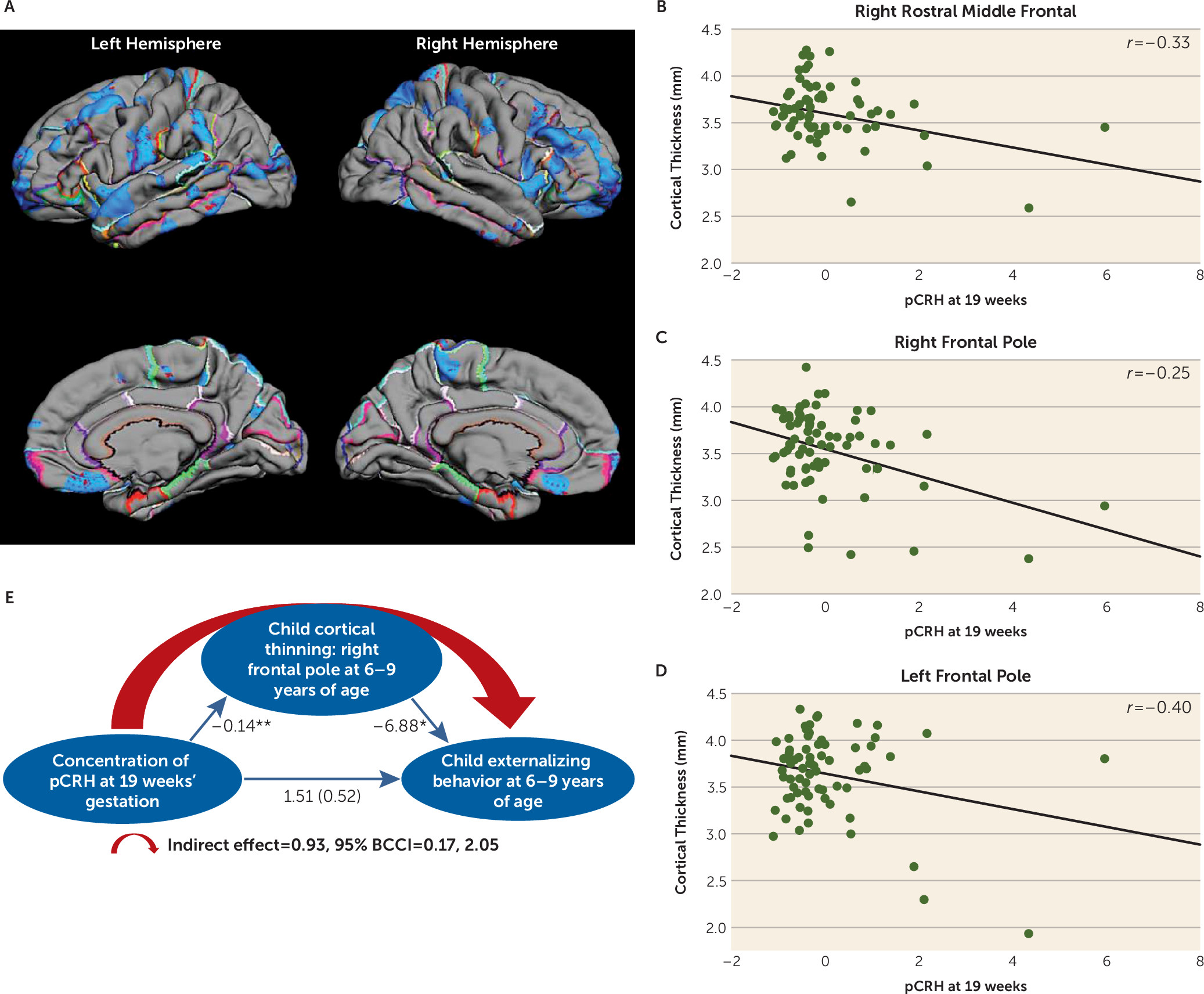
CRH Levels Early in Gestation: Regional Cortical Thinning and Behavioral Outcomes
Fetal exposure to pCRH early in gestation (19 weeks) was associated with significant thinning of the frontal poles. The significant association between pCRH and cortical thinning was bilateral but strongest in the right (B=−0.14, p<0.001; 78% of the structure) compared with the left frontal pole (B=−6.88, p<0.05; 69% of the structure) (see Table S3 in the
data supplement). In
Figure 2, pial maps depict areas of significant thinning linked with fetal pCRH exposure early in gestation, and the scatterplots illustrate the regions of the frontal cortex with the strongest associations. (Findings were essentially unchanged with log transformed pCRH values; see Table S5 in the
data supplement.) Nearly identical associations were observed in a smaller subsample (N=57) of children exposed to high pCRH levels measured even earlier, at 15 weeks’ gestation (see Figure S3A–D in the
data supplement).
Thinning of the frontal pole has been associated with externalizing behaviors (
50), defined as actions that direct energy outward and tend to harm others (
51). We tested whether the effects of pCRH exposure on cortical thinning contributed to the development of these behaviors (
Figure 2E). Our model supported the belief that reduced cortical volume in the frontal pole, associated with elevated fetal exposure to concentrations of pCRH at 19 weeks’ gestation, contributes to externalizing symptoms in 6- to 9-year-old children (indirect effect=0.93; 95% bias-corrected confidence interval [BCCI]=0.17, 2.05; p<0.05). Neither internalizing problems nor reaction time on the flanker task was associated with cortical thinning in this region.
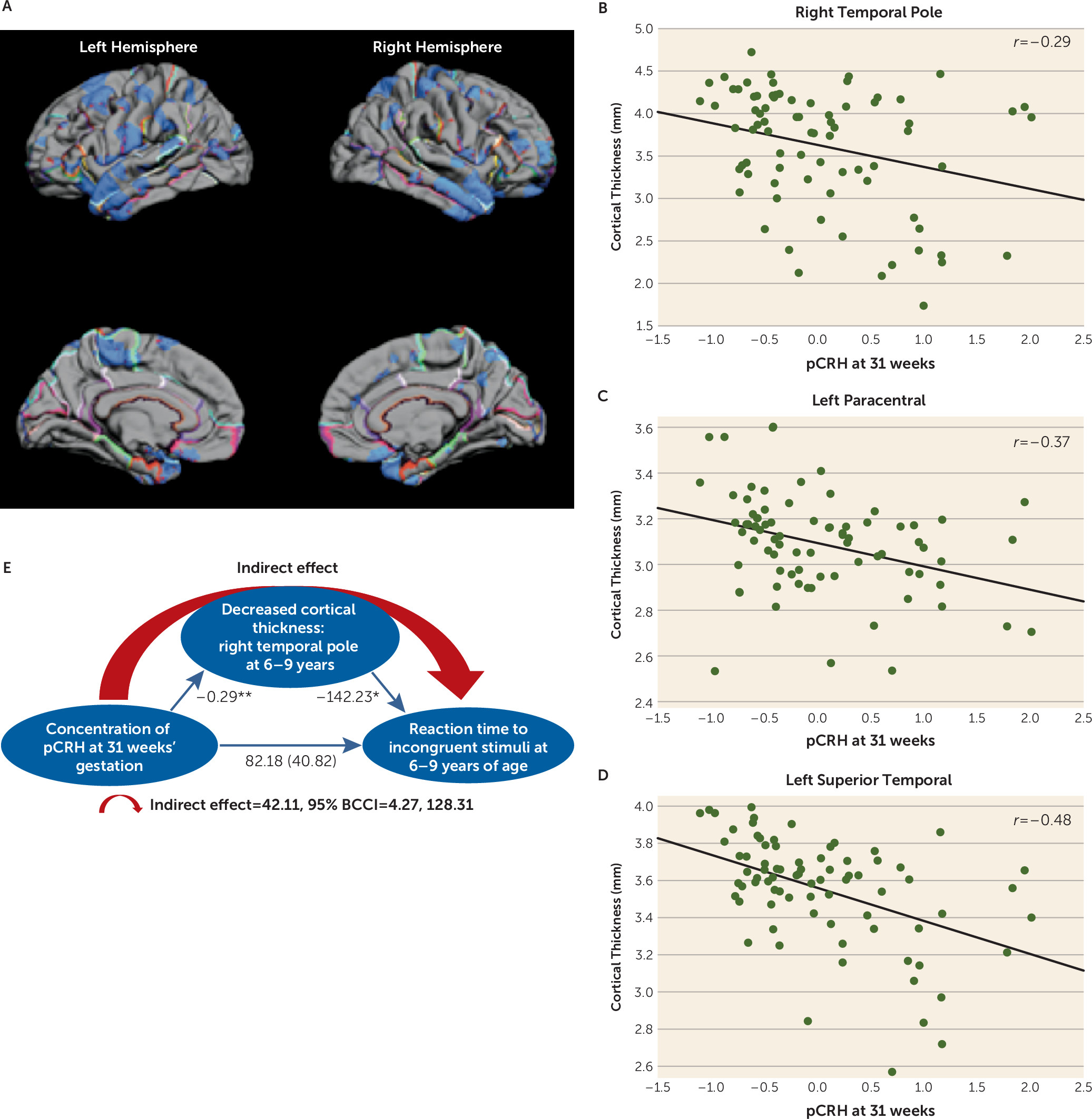
CRH Levels in Late Gestation: Regional Cortical Thinning and Cognitive Outcomes
Exposure to elevated levels of pCRH later in gestation (31 weeks) was associated with cortical thinning in the lateral temporal and paracentral regions (
Figure 3A). The effect was localized to the left paracentral region and was bilateral in the temporal cortex. Remarkably, 98% of the lateral surface of the right temporal pole and 66% of the left temporal pole was significantly thinner in children exposed to high levels of pCRH at 31 weeks’ gestation (see Table S4 in the
data supplement). The scatterplots in
Figure 3 illustrate these regional associations. (Similar associations were observed at 25 weeks’ gestation in a smaller subsample [N=50].)
Structures comprising the temporal cortex subserve numerous cognitive and emotional functions. Temporal cortical thinning has been reported in individuals with attentional deficits (
52), especially in the right temporal pole (
53), which is active in tasks requiring attention to relevant stimulation (
54). We employed a statistical model (
55) to test whether the effects of exposure to pCRH late in prenatal life on cortical thinning contributed to impairment of attention in 6- to 9-year-olds. The model (
Figure 3E) indicated that the reduced right temporal pole volume associated with exposure to pCRH at 31 weeks’ gestation may partially account for poorer performance on a visual processing and sustained attention test (indirect effect=42.11; 95% BCCI=4.27, 128.31; p<0.01) (
56,
57).
Sex Differences
Exploratory analyses suggest significant sex differences in the nature and degree of association between pCRH levels and global as well as regional cortical thinning. At both 19 and 31 weeks’ gestation, the associations were stronger in girls (see Figure S2 in the data supplement). The association between fetal exposure to pCRH at 19 weeks’ gestation and cortical thinning involved most cortical areas in girls (see Figure S2A) but minimal areas in boys (see Figure S2B). Fetal exposure to pCRH at 31 weeks affected cortical thinning globally in boys (see Figure S2C) but locally in the temporal pole in girls (see Figure S2D; similar to the findings for the combined sexes).
Discussion
The principal and novel findings in this series of studies are that human fetal exposure to pCRH, even at concentrations that are insufficient to initiate labor or early delivery, is associated with regional cortical thinning and commensurate cognitive and emotional problems in a sex-specific manner in school-age children. Notably, such problems often are prodromal events that are associated with eventual neuropsychiatric outcomes.
The human placenta expresses pCRH by the eighth week of gestation, and concentrations of pCRH increase geometrically as pregnancy advances to regulate the timing and onset of labor and delivery (
30,
58). Indeed, extremely high levels of pCRH, often a result of adverse events during pregnancy, stimulate a cascade of events that results in preterm labor and delivery. Premature birth is a significant contributing factor to impaired neurological and psychiatric outcomes. However, pCRH levels also rise in response to a variety of maternal stresses, and the novel finding here is the profound consequence of fetal exposure to pCRH at levels that are not associated with early delivery in full-term school-age children. Specifically, we identify pronounced thinning in discrete cortical areas with implications for emotional and cognitive functions. Pronounced cortical thinning was associated with fetal exposure to pCRH across gestation, with some evidence of localization in the prefrontal and temporal poles. Examination of discrete gestational intervals clarified the fact that prefrontal thinning was associated with exposure at early midgestation, and thinning of the temporal pole was associated with exposure later in gestation.
Our findings raise the novel possibility that CRH is causally linked to cortical thinning rather than merely signifying the presence of a harsh, stressful milieu for the developing fetal brain. A causal role for CRH in cortical thinning is supported by recent findings in an animal model (
22). Cortical volume is largely made up of dendritic trees of cortical neurons (
22,
59), and changes in dendritic arborization can be measured as volume loss in MR imaging (
60). Our recent experiments (
22) demonstrate that exposure of developing cortical neurons to physiological levels of CRH results in a dose-dependent reduction and impoverishment of dendritic arborization. In addition, exposure to early postnatal CRH in vivo (
61) and to maternal stress signals (
62,
63) both provoke similarly impoverished dendritic trees in the hippocampus. Indeed, exposure to nanomolar levels of CRH has been shown to reduce dendritic length and complexity via CRH receptor type 1 (
64), which is expressed on the dendrites (
65,
66). Eliminating the actions of endogenous CRH, both in transgenic mice lacking the CRH receptor and via chronic exposure of organotypic slice cultures to CRH receptor blockers (
64,
65), has been shown to lead to exuberant dendritic trees. Thus, it appears that the role of physiological levels of CRH is to modulate—perhaps in concert with glucocorticoids (
67,
68)—neuronal dendritic development in the perinatal hippocampus and neocortex.
The sex differences observed here are intriguing and consistent with increased prevalence of stress-related disorders in women compared with men—tendencies also observed in prepubertal children (
69). Our novel findings here suggest that sex-specific structural consequences of early-life adversity and potentially of CRH may be most apparent in females and are consistent with conclusions that females exposed to prenatal stress are more likely than males to exhibit increased levels of anxiety, impaired executive function, and neurological markers associated with these behaviors (
69).
In summary, our findings uncover a novel and unexpected result of prenatal elevation of pCRH: reduction in cortical volume in typically developing children, perhaps related to stunting of normal neuronal growth, and consequent commensurate subtle but significant emotional and cognitive impairments. The behavioral assessments were not designed a priori for assessing the unexpected cortical thinning observed in the temporal lobes, and this limitation should be addressed in future studies. Even when CRH levels are not sufficient to trigger premature birth and children are born at term, fetuses exposed to increased levels of pCRH carry less well developed cortical neurons, apparent from extensive yet selective areas of cortical thinning. The cortical thinning is biologically significant because it is associated with both cognitive and emotional deficits.
Acknowledgments
The authors gratefully acknowledge the assistance of Claudia Buss and Mariann Howland.