Antipsychotic drugs, the first-line treatment for schizophrenia, act through the antagonism of central dopamine D
2 receptors (
1). Although they are effective in most patients, therapeutic response is poor in up to one-third of patients (
2); this may reflect an absence of elevated dopamine function in this subgroup (
3). In patients who respond to antipsychotics, the beneficial effects are mainly on positive psychotic symptoms: antipsychotics have relatively little impact on negative symptoms and cognitive impairments (
4). This may be because, in contrast to positive symptoms, these features of schizophrenia are not driven by elevated dopamine function (
2). Compounds whose molecular mechanism of action differs from that of antipsychotic drugs might therefore improve the treatment of schizophrenia.
The two most abundant cannabinoids in
Cannabis sativa are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Different forms of cannabis vary in the relative proportions of THC and CBD, and the risk of psychotic symptoms and impaired cognitive functioning following cannabis use is lower with cannabis preparations that have a relatively high CBD content (
5). In healthy volunteers, pretreatment with CBD attenuates both the experimental induction of psychotic symptoms by THC (
6,
7) and the adverse effects of THC on cognitive performance (
8–
10). The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors (
11).
The first evidence that CBD might be useful in treating schizophrenia came from a case report in which it was found to improve symptoms in a patient who had not responded to haloperidol (
12).
CBD has also been reported to reduce psychotic symptoms in patients with Parkinson’s disease (
13). The only previous trial of CBD in schizophrenia compared CBD against amisulpride in patients with an acute exacerbation of schizophrenia symptoms (
14). After 4 weeks of treatment, both drugs had reduced ratings on the Positive and Negative Syndrome Scale (PANSS) to a similar extent, but CBD was associated with fewer adverse effects.
The aim of the present study was to explore the safety and effectiveness of CBD as an adjunctive treatment in schizophrenia. In a randomized double-blind trial, patients who had been partially responsive to antipsychotic medication received either CBD or placebo as an add-on treatment for 6 weeks. We examined the effects of CBD on positive and negative psychotic symptoms, cognitive performance, level of functioning, and the treating psychiatrist’s overall clinical impression.
Method
Study Design
This phase 2, 8-week, multicenter, double-blind, randomized, placebo-controlled, parallel-group trial was conducted at 15 hospital sites in the U.K., Romania, and Poland. The trial was approved by the relevant institution review board or ethical committee in each country and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Tripartite Guideline on Good Clinical Practice. All patients provided written informed consent.
Participants and Procedures
Consenting patients were screened at visit 1. Those who met the inclusion criteria underwent randomized treatment assignment the same day. Participants had to be 18–65 years of age and have schizophrenia or a related psychotic disorder as defined by DSM-IV. They were required to have previously demonstrated at least a partial response to antipsychotic medication (i.e., they did not have treatment-resistant illness) and to have been receiving a stable dosage of antipsychotic medication for at least 4 weeks; this treatment was continued unchanged for the duration of the trial. Exclusion criteria included a PANSS score <60 at screening, taking more than one antipsychotic medication, or presence of delirium, dementia, or any disorder or clinical finding that, in the opinion of the investigator, may have put the patient at risk by taking part in the trial, may have influenced the results, or may have limited the patient’s ability to participate. Substance use was not an exclusion criterion, and use of alcohol, cannabis, or other substances during the study was not prohibited. However, patients in whom psychosis may have been induced by substance use were excluded. Patients who were pregnant, lactating, or planning pregnancy during or within 3 months of the completion of the trial were also excluded.
After randomization, baseline assessments of symptoms, general functioning, cognitive performance, and extrapyramidal signs were conducted, and the patient’s weight, body mass index (BMI), and waist circumference were recorded. Current substance use was assessed using a semistructured interview. Blood was collected for assessment of levels of high-density lipoprotein (HDL) cholesterol, liver enzymes, prolactin, inflammatory markers, CBD, and CBD metabolites (for the schedule of assessments, see Table S1 in the data supplement that accompanies the online edition of this article).
Patients were reassessed using the same measures on days 8, 22, and 43 (±3 days), and the clinician’s impression of severity and improvement in the patient’s condition and patient-reported or caregiver-reported impressions of general functioning and sleep were recorded. Current substance use was reassessed at the end of treatment.
Concomitant medications and any adverse events were reviewed at all visits. A safety follow-up telephone call was made on day 57.
Randomization and Masking
Patients were randomly assigned in a 1:1 ratio to receive 1,000 mg/day of CBD (10 mL of a 100 mg/mL oral solution) or matching placebo (excipients alone), administered in two divided doses (morning and evening). The randomization schedule was produced by an independent statistician, held centrally and not divulged to anyone involved in the trial. A unique treatment number was used to identify each carton of investigational medicinal product and the bottles it contained; CBD and placebo cartons and bottles were identical in appearance. After randomization, patients were allocated an investigational medicinal product pack in sequential treatment number order. Adherence was evaluated by measuring the volume of investigational medicinal product used between study visits and by measuring plasma CBD levels at baseline and at the end of treatment. None of the participants or investigators reported that they could distinguish between the CBD and placebo preparations or could guess the treatment group on the basis of therapeutic response or adverse effects, although this was not assessed systematically.
Outcomes
Because the trial was exploratory, a number of key endpoints were defined, rather than a single primary endpoint. Key endpoints were related to symptom severity, level of functioning, and cognitive performance. They included PANSS total, positive, negative, and general scores and responder analysis (see below); Scale for the Assessment of Negative Symptoms (SANS) score; improvement score on the Clinical Global Impressions Scale (CGI-I); Global Assessment of Functioning (GAF) scale score; and the composite score on the Brief Assessment of Cognition in Schizophrenia (BACS).
Other measures included changes in the severity scale of the CGI (CGI-S); the functioning and sleep scales of the Participant and the Carer Global Impression of Change Scale; the Simpson-Angus Scale; body weight, waist measurement, BMI, and HDL cholesterol levels.
The safety and tolerability of CBD were monitored through the assessment of adverse events, clinical laboratory tests, and vital signs.
Statistical Analysis
Primary analyses were based on data in the intention-to-treat analysis set. The change in PANSS total score from baseline to end of treatment (using the last observation carried forward) was analyzed using analysis of covariance, using age and baseline PANSS positive, negative, and general scores as covariates. The number of responders (defined as patients who had an improvement ≥20% in PANSS total score) per treatment group was analyzed using logistic regression, with age and baseline PANSS positive, negative, and general scores as covariates. After unblinding of the data, a post hoc analysis of the number of responders in each treatment group (with response defined as an improvement ≥20% in PANSS positive score) alone was conducted.
Other outcome measures were quantified as change from baseline score and analyzed in a way similar to the PANSS total score.
All statistical tests were two-sided at the 5% significance level.
Sample Size
Allowing for a 20% dropout rate, a sample size of 78 patients (39 per group) was calculated as sufficient to detect a treatment difference of 11 in PANSS total score between CBD and placebo on the change from baseline to end of treatment, assuming a standard deviation of 15, with a two-tailed 5% significance level and 80% power.
Results
Of 89 patients screened, 88 underwent randomized assignment to treatment across 15 sites. Five patients subsequently withdrew from the trial, two because of adverse events and three because of withdrawal of consent, leaving 83 patients who completed the trial (a patient flow chart is available in the data supplement that accompanies the online edition of this article). Of the 88 patients randomized, 11 were recruited from sites in the U.K., 37 from sites in Poland, and 40 from sites in Romania. Most participants (N=83) had a DSM diagnosis of schizophrenia; the diagnoses in the remaining patients were of schizoaffective disorder (N=3), schizophreniform disorder (N=1), and delusional disorder (N=1).
Before unblinding, one patient in the CBD group was identified as having no postbaseline efficacy data, and one patient in the placebo group had a PANSS total score <50 during screening; both patients were excluded from the intention-to-treat analysis set. Another four patients (three in the CBD group and one in the placebo group) received the investigational medicinal product for ≤21 days; their data were excluded from the per protocol analysis set.
Baseline Patient Characteristics
Baseline patient characteristics were similar between the treatment groups (
Table 1). One patient in the CBD group and two in the placebo group tested positive for THC at baseline, but this was not an exclusion criterion. Because so few patients tested positive for THC, it was not possible to assess whether the effects of CBD were influenced by cannabis use.
Throughout the trial, all patients remained on the same oral antipsychotic medication they had been taking before the study, at the same dosage. The most common medications were aripiprazole, olanzapine, and risperidone (see Table S2 in the online data supplement).
Key Endpoints
Symptom severity scale scores.
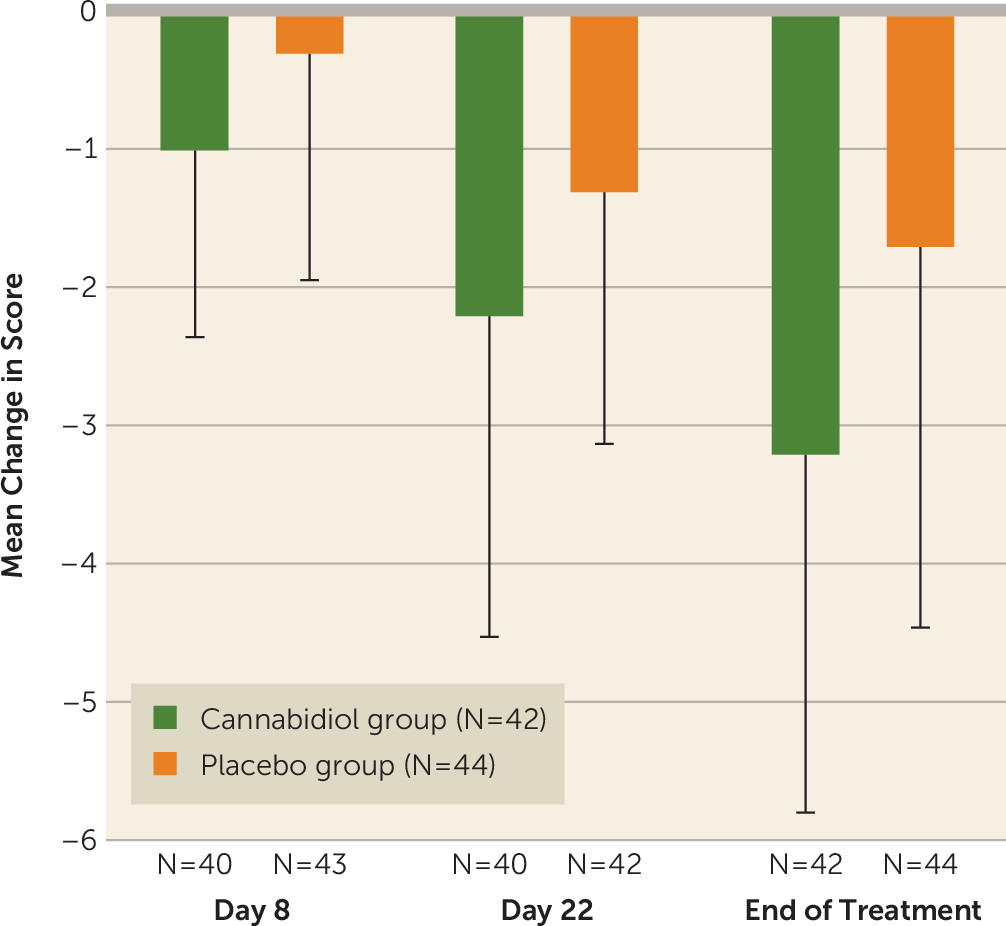
Positive psychotic symptoms (measured using the PANSS positive subscale) were significantly reduced from baseline to end of treatment in the CBD group compared with the placebo group (
Table 2,
Figure 1). Changes in the levels of negative psychotic symptoms (using the SANS and the negative subscale of the PANSS), overall psychopathology (PANSS total score), and general psychopathology (PANSS general subscore) over the treatment period were not significantly different between the CBD and placebo groups (
Table 2).
The proportion of treatment responders (patients with an improvement ≥20% in PANSS total score) was higher in the CBD group than in the placebo group, although the number of responders per group was small (12 and six patients, respectively) and the difference fell short of statistical significance (odds ratio=2.62, 95% CI=0.86, 8.00; p=0.09). A similar trend was evident when treatment response was defined in terms of improvement in the PANSS positive score (odds ratio=2.49, 95% CI=0.98, 6.38; p=0.056).
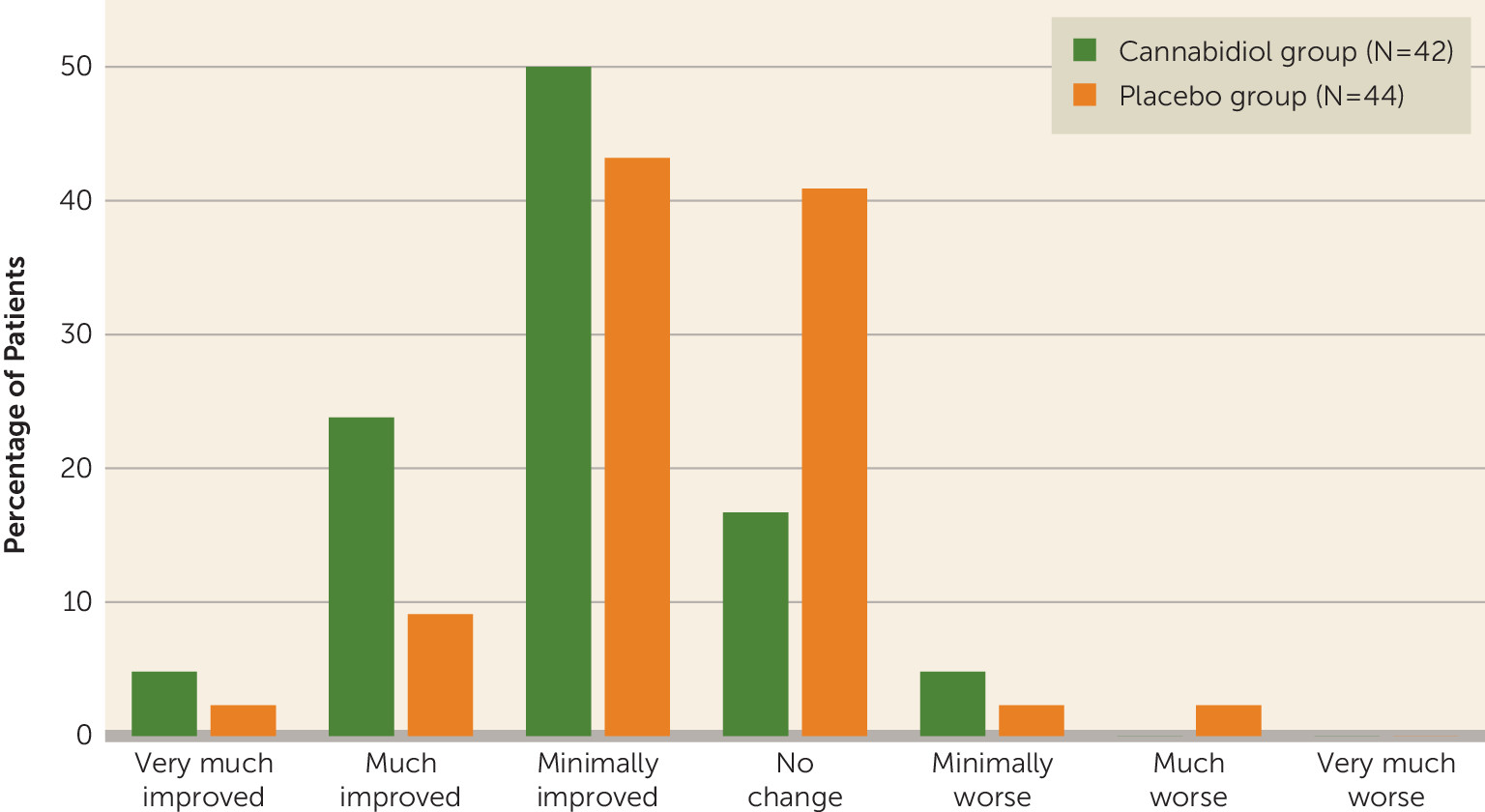
CGI improvement and severity ratings.
At the end of treatment, a significantly higher proportion of patients in the CBD group were rated by their clinician as “improved” on the CGI-I scale compared with those in the placebo group (78.6% and 54.6%, respectively; treatment difference=−0.5, 95% CI=−0.8, −0.1; p=0.018). The CBD group had higher proportions of patients in all three of the improvement subcategories (very much improved, much improved, and minimally improved) compared with the placebo group (
Figure 2).
At baseline, the distribution of CGI-S scores in the two treatment arms was similar, with the majority of patients in both groups classed as moderately, markedly, or severely ill. By the end of treatment, the proportion of patients in these three categories had decreased from 83.4% to 54.8% in the CBD group and from 79.6% to 63.6% in the placebo group. This corresponded with an increase in the proportion of patients classed as having mild, borderline, or no illness from 16.7% to 45.2% in the CBD group, and from 20.5% to 36.4% in the placebo group. These group differences in longitudinal change in CGI-S score were significant (treatment difference=−0.3, 95% CI=−0.5, 0.0; p=0.044).
Level of Functioning
At baseline, the GAF scores were similar between the CBD and placebo groups, but by the end of treatment the CBD group showed a greater improvement, although the difference fell short of statistical significance (treatment difference=3.0, 95% CI=−0.4, 6.4; p=0.08) (see Table S3 in the online data supplement). There were no significant group differences in patient and carer impressions of change in general functional ability.
Cognitive Performance
At baseline, the BACS composite scores were similar in the CBD and placebo groups (32.21 and 32.91, respectively). There was a greater improvement in the BACS composite score in the CBD group, although it fell short of significance (treatment difference=1.31, 95% CI=−0.10, 2.72; p=0.068). Post hoc analysis of the individual BACS domains showed that there was a significantly greater improvement in motor speed in the CBD group relative to the placebo group (p<0.05), and a nonsignificantly greater improvement in executive functions (p=0.068) (see Table S4 in the online data supplement).
Other Measures
There were no significant changes in prolactin levels, Simpson-Angus Scale ratings, weight, waist circumference, liver function tests, inflammatory markers, or HDL cholesterol levels in either group, and no between-group differences. There were no group differences in the quality or quantity of sleep. At baseline, one patient in the CBD group was cannabis dependent (two joints per night); the patient’s pattern of use did not change during the study. Another patient in the CBD group was dependent on alcohol at baseline but not at the end of treatment.
At baseline, low but quantifiable plasma levels of CBD and/or its hydroxyl metabolites (6-OH-CBD or 7-OH-CBD) were detected in two patients (5%) in the CBD group and in five patients (11%) in the placebo group. At the end of treatment, quantifiable plasma levels of CBD and/or its hydroxyl metabolites were detected in all 41 of the patients in the CBD group, and in two of the 43 patients (5%) in the placebo group (although the levels were again low). Within the CBD group, there was no significant correlation between plasma levels of CBD or its metabolites and the magnitude of PANSS and SANS treatment responses.
Adverse Events
There were 30 reported treatment-emergent adverse events in 15 patients in the CBD group and 35 in 16 patients in the placebo group. Ten patients in each group reported at least one event that was classed as treatment related. Gastrointestinal events were the most common and were reported by nine patients in the CBD group and three in the placebo group (
Table 3). In both groups, most events (80% and 81%) were mild and resolved without intervention (83% and 80%). Two patients in the CBD group experienced a treatment-emergent adverse event that resolved with sequelae: mild lowered blood pressure and moderate chest pain; neither was considered treatment related. Ten treatment-emergent adverse events were still ongoing at the end of the trial, reported by three patients in the CBD group (three events) and four in the placebo group (seven events). Only two events in the CBD group were considered to be treatment related (dyslipidemia and nausea); both were mild.
One serious treatment-emergent adverse event was reported: a clinical exacerbation of schizophrenia in a patient in the placebo group. This event was considered to be unrelated to the trial treatment, and it had resolved by the end of the study. One patient in each group withdrew because of treatment-emergent adverse events (see Figure S1 in the online data supplement). The withdrawal from the CBD group followed nausea, diarrhea, abdominal pain, and vomiting, and the withdrawal from the placebo group was due to somnolence and altered perception. In both cases, these symptoms subsequently resolved.
Discussion
This is, to our knowledge, the first placebo-controlled trial of CBD in schizophrenia. The data indicate that 6 weeks of treatment adjunctive to antipsychotic medication was associated with significant effects both on positive psychotic symptoms and on the treating clinicians’ impressions of improvement and illness severity. There were also improvements in cognitive performance and in the level of overall functioning, although these fell short of statistical significance.
Although the magnitude of the effect on positive symptoms was modest, it was seen in patients who were already being treated with antipsychotic medication at appropriate dosages; the improvement was thus over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that there was an improvement that was evident to the treating psychiatrists and may therefore be clinically meaningful (
15). These findings are consistent with previous evidence that CBD can reduce psychotic symptoms in schizophrenia (
11,
13), in Parkinson’s disease (
12), and in THC-induced psychosis (
6).
The trend for an overall improvement in cognitive performance raises the possibility that CBD may have beneficial effects on cognition. Post hoc analyses indicated that the strongest improvements were in motor speed and executive functioning. Only one previous study has assessed the effects of CBD on cognitive function in schizophrenia, examining the effect of a single dose on performance on the Stroop task (
16). However, several studies in healthy volunteers have shown that CBD attenuates the acute impairment of memory function that follows administration of THC (
5,
8–
10). The possibility that CBD could have beneficial effects on cognitive function in schizophrenia merits further investigation.
Only one-third of the patients in each group reported treatment-emergent adverse events. The majority of these were mild and resolved spontaneously, with no significant differences in frequency between the CBD and placebo groups. This is in line with previous observations in volunteers (
17,
18), patients with schizophrenia (
14,
16,
19), and patients with other disorders (
13,
18,
20), all of which indicate that CBD has a favorable tolerability profile. This is a major potential benefit for a candidate treatment for schizophrenia; antipsychotic medication can be associated with adverse effects that may reduce adherence and contribute to the poor physical health of patients with the disorder (
21).
Although cannabis use is common in schizophrenia, only a few patients in our sample tested positive for THC. While this indicates that it is unlikely that the findings were related to cannabis use, the extent to which the effects of CBD in schizophrenia are influenced by concurrent or previous cannabis use merits further investigation.
The effects of CBD do not appear to depend on dopamine D
2 receptor antagonism. A number of mechanisms of action have been proposed, including inhibition of fatty acid amide hydrolase (
13), inhibition of adenosine reuptake (
22), TRPV1 and 5-HT
1A receptor agonism, and D2High partial agonism (
23). Because CBD acts in a way different from conventional antipsychotic medication, it may represent a new class of treatment for schizophrenia. However, its potential clinical utility will require further investigation in larger-scale trials.