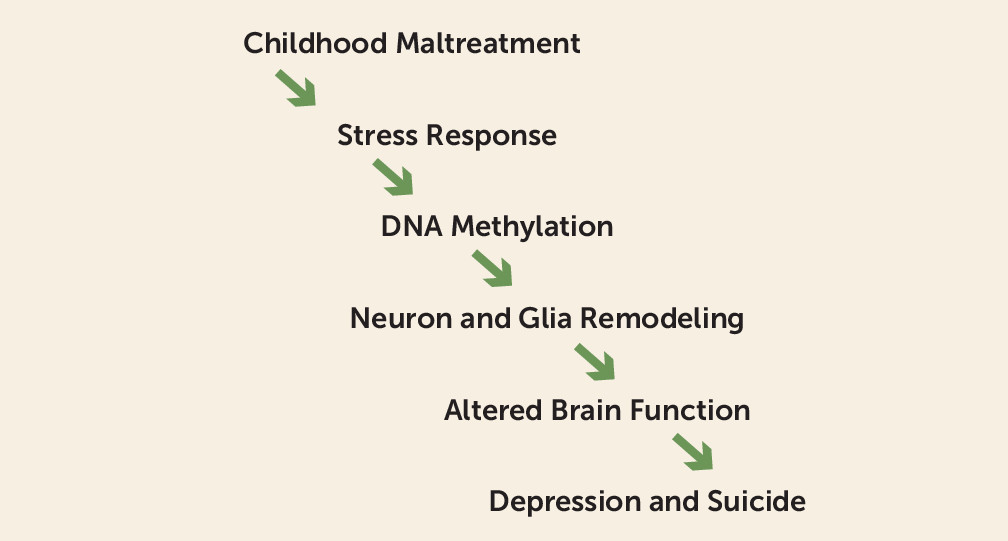
Childhood maltreatment—sexual abuse, physical abuse, psychological abuse, and parental neglect—is associated with increased vulnerability to developing psychopathology as an adult. Indeed, childhood maltreatment is one of the strongest predictors of adult depression and suicide (
1,
2). Although the psychiatric outcomes associated with childhood maltreatment are well characterized, the neurobiological mechanisms underlying the associations are largely unknown. Elucidating the neurobiological mechanisms by which childhood maltreatment increases risk for adult psychopathology is of critical importance for developing effective and specific prevention and treatment strategies. Over the past decade, epigenetic programming has been advanced as a neurobiological mechanism that may explain the link between childhood maltreatment and vulnerability to adult psychopathology (
3,
4).
Epigenetic programming describes the process through which an environmental stimulus during development alters epigenetic marks (e.g., DNA methylation, histone modifications, etc.) and thus gene expression in a way that reshapes the developmental trajectory and produces persistent phenotypic effects (
5). DNA methylation, a major epigenetic mark, is the addition of a methyl group at the five-carbon position of cytosine nucleotides in DNA. Changes in DNA methylation near gene regulatory regions affect local gene expression. Epigenetic programming is an attractive candidate mechanism for mediating the link between childhood maltreatment and adult psychopathology, as patterns of DNA methylation are progressively established during brain development, and these patterns are altered by early-life adversity (
6). Studies have shown robust DNA methylation alterations in individuals with a history of childhood maltreatment, but there is little understanding of how these alterations might lead to increased vulnerability to adult psychopathology. In this issue of the
Journal, Lutz et al. (
7) test the hypothesis, broadly summarized in
Figure 1, that epigenetic programming is the mechanism by which childhood maltreatment leads to an increased vulnerability to adult psychopathology.
In a cohort of postmortem brains, Lutz et al. used epigenetic, transcriptional, and anatomical approaches to investigate the anterior cingulate cortex—a brain region that imaging studies have consistently found to exhibit childhood maltreatment–related alterations in structure, connectivity, and function (
8). Uniquely, this cohort comprised individuals for whom early-life histories, including experiences of sexual and physical abuse, had been characterized using standardized psychological assessment tools. Using reduced representation bisulfite sequencing and RNA sequencing approaches, the investigators first performed genome-wide screenings of DNA methylation and gene transcription in three groups: individuals who committed suicide and had a history of childhood maltreatment, individuals with depression who committed suicide, and individuals without any psychiatric diagnoses or history of childhood maltreatment. These first screens were performed in gray matter and thus included multiple different cell types. Interestingly, three of the genomic regions in which DNA methylation was most different between the groups with and without a history of childhood maltreatment intersected genes directly related to myelin and oligodendrocytes (LINGO3, POU3F1, and ITGB1), and myelin- and oligodendrocyte-related genes were strongly enriched for genes differentially transcribed in individuals with a history of childhood maltreatment. This observation led the authors to hypothesize that childhood maltreatment may affect oligodendrocytes selectively. To test this hypothesis, they performed targeted bisulfite sequencing of the three genes in nuclei from oligodendrocyte-lineage cells and neurons separately and found LINGO3 and POU3F1 to be hypomethylated in oligodendrocytes, but not neurons, in the group with a history of childhood maltreatment. Notably, the childhood maltreatment–associated molecular alterations were not found in the group with depression who committed suicide but did not have a history of childhood maltreatment, thus suggesting that they are not accounted for by psychopathology.
Next, to connect the childhood maltreatment–associated molecular alterations with cellular alterations, the authors assessed oligodendrocyte and myelinated axon morphology. They found that childhood maltreatment is associated with a reduction in the thickness of axons and their myelin sheaths selectively in small-diameter axons, a finding that suggests that alterations in the morphology of oligodendrocytes and their myelination may serve as substrates for mediating the long-term consequences of childhood maltreatment.
Had the authors concluded their study at this point, they would have been able to point to some important molecular and cellular changes that correlate with childhood maltreatment in postmortem human brain tissue, and this would have represented a significant contribution. However, as is the case with the best translational studies that capitalize on postmortem brain tissue, Lutz et al. sought to complement their findings in a model system. They chose to study gene transcription in a rodent model that captures some features of neglectful parenting (
9). They found that offspring of dams that provided low levels of maternal care exhibited a gene transcription profile similar to that seen in the human subjects with history of childhood maltreatment (i.e., changes in myelin-related transcripts). Although these findings certainly strengthen the notion that transcription of oligodendrocyte- and myelin-related genes is sensitive to early-life adversity, cell-type-specific DNA methylation data would have more convincingly supported their overall narrative.
Although this study significantly expands our knowledge of the molecular and cellular sequelae associated with childhood maltreatment, our understanding of epigenetic programming and other mechanisms that may link childhood maltreatment and vulnerability to adult psychopathology remains quite limited. This study, however, does support a role for epigenetic programming, and further work to understand this putative mechanism is in order. To develop our understanding of epigenetic programming to a degree that effective and specific mechanism-based prevention and treatment strategies are conceivable, the next generation of studies will need to accomplish two goals: 1) a detailed characterization of the associations among childhood maltreatment, epigenetic marks, and adult psychopathology and 2) an understanding of which, if any, of the epigenetic alterations associated with childhood maltreatment and vulnerability to adult psychopathology are causal.
It is toward the first goal that the Lutz et al. study advances our understanding. Further advances toward understanding how childhood maltreatment affects the epigenomic landscape of neurons and glia will require building on and extending the approaches used in this study. A feature of the study that was particularly powerful was the use of a postmortem brain cohort that was well characterized with respect to childhood experiences, including childhood maltreatment. Childhood maltreatment, however, is a broad term and includes sexual and physical abuse as well as parental neglect, and each of these experiences, as well as the developmental epoch in which they occur, is likely to be associated with particular epigenetic alterations and psychiatric outcomes. Additionally, the epigenetic alterations induced by each of these experiences will need to be described in a cell-type-specific manner and in multiple brain regions. One lesson from the present study is that the affected cell type is not necessarily predictable a priori.
Despite the great value in studying the epigenetic alterations in postmortem brains from individuals with a history of childhood maltreatment, such studies have limitations. One limitation is the fact that the brains were from individuals who each had a lifetime of unique experiences (and a unique complement of genetic variation), any of which could conceivably have an impact on the epigenomic landscape in, so far, unpredictable ways. Thus, animal models in which nature, dose, and timing of environmental exposures can be controlled and genetic influences accounted for will be required to actualize this goal. Of course, modeling uniquely human phenomena, such as childhood maltreatment, has its own limitations. Such a limitation is exemplified by the use of a rodent model of maternal neglect in the present study on human subjects with history of childhood maltreatment defined as physical and sexual abuse. This is of course a reasonable approach given the absence of a more appropriate option, but it is nonetheless an imperfect model. Progress toward this goal will no doubt be aided by the pace at which genomic and epigenomic methods are evolving, with increasingly high throughput and low cost.
Actualizing the second goal—determining whether the epigenetic alterations associated with childhood maltreatment and vulnerability to later psychopathology are causal—will depend on the maturing of existing genomic and epigenomic tools. Until recently, establishing causal links between epigenetic alterations and phenotypes seemed fantastical. The ability to establish causality was limited by the lack of tools that could selectively manipulate the epigenome. In the past several years, however, such epigenomic editing tools have become a reality. A common feature of these tools is that they employ unique nucleotide sequences to target secondary effector proteins that are capable of robust epigenetic modification. These tools are based on zinc-finger nucleases, transcriptional-activator-like effectors, and clustered regularly interspaced short palindromic repeats (CRISPR) interacting with CRISPR-associated protein 9 (Cas9) nucleases. Tools based on the CRISPR-Cas9 system are the newest, and they offer some advantages over the others. One CRISPR-Cas9-based tool that has been used for targeted DNA methylation in in vitro cell models has two parts: 1) a fusion protein comprising an inactive Cas9 (dCas9) endonuclease for targeting a specific genomic region and the DNA methyltransferase 3A catalytic domain for methylating local cytosines, and 2) a guide RNA, which binds both dCas9 and genomic regions complementary to its
∼20 nucleotide “targeting” sequence (
10). A system like this could be used in conjunction with genomic editing tools (e.g., chemically inducible expression systems, optogenetics, and tissue-specific promoters) to manipulate the epigenome with genetic, cellular, and temporal precision (
11).
These tools will be able to be used initially in in vitro cell models to assess how specific epigenetic alterations found to be associated with childhood maltreatment affect gene expression, the dynamic epigenomic landscape, and cell function. For example, childhood maltreatment–associated DNA methylation alterations observed in myelin- and oligodendrocyte-related genes could be modeled in an oligodendrocyte cell culture using the CRISPR-Cas9-based tool described above. The transcription of myelin- and oligodendrocyte-related genes as well as neurite and myelin sheath thickness could subsequently be measured in these cultured oligodendrocytes to assess how closely they recapitulate those observed in the human postmortem brain study. As the epigenomic editing tools mature and are moved into animal models, the effect of childhood maltreatment–associated epigenetic alterations on in vivo gene expression, cell function and morphology, circuitry, and psychopathology-related neurophysiology and behaviors will be able to be measured. Such tools will also improve the prospects of DNA methylation as a therapeutic target for disease states and even for reversing the effects of negative environmental exposures like childhood maltreatment.
In summary, the results of the Lutz et al. study provide the most detailed and comprehensive description to date of a potential molecular and cellular mechanism by which childhood maltreatment causes long-term structural and functional changes that may give rise to an elevated vulnerability to adult psychopathology. The convergence of findings like these with maturing genomic and epigenomic methods gives new hope that the promise of epigenomic therapeutics in psychiatry can be translated into a reality.