Corticotropin-releasing hormone binding protein (CRHBP) plays a modulatory role in regulating the function of the canonical stress-responsive hypothalamic-pituitary-adrenal (HPA) axis. It is also found throughout the brain, where it functions to regulate the availability of neuronally released corticotropin-releasing hormone (CRH) in relation to the activation of CRH receptors located in extrahypothalamic brain regions. In this issue of the
Journal, O’Connell et al. (
1) report a novel and potentially important finding demonstrating that variation in the CRHBP gene (single-nucleotide polymorphism [SNP] rs28365143) predicts effective antidepressant treatment response. This effect was selective to serotonin reuptake inhibitor (SSRI) treatment and was not observed in relation to serotonin-norepinephrine reuptake inhibitor treatment. While much additional work and validation needs to be performed, these findings point to the possibility that genetically determined differences in the function of CRHBP are mechanistically involved in mediating the antidepressant effects of SSRI antidepressants. CRHBP is an important member of the CRH system, but it has been relatively understudied in relation to psychopathology, and this finding adds to others suggesting that more in-depth studies of CRHBP may be fruitful.
Since the early work of Selye (
2), cortisol and the HPA axis have been associated with adaptive and maladaptive responses to stress. In response to stress, CRH is released from neurons in the paraventricular nucleus of the hypothalamus, which stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, which in turns stimulates the secretion of cortisol from the adrenal cortex. For years, evidence has accumulated linking various alterations in the function of the HPA axis to depression. Early studies focused on cortisol, and, after the characterization of CRH by Vale in 1981 (
3), researchers focused intensively on CRH as a possible candidate underlying the pathophysiology of depression and accompanying HPA alterations.
Subsequent basic research established that CRH was found not only in the hypothalamus, where it functions to stimulate the release of ACTH, but also throughout the brain in key regions that are involved in mediating behaviors, emotions, and physiological responses (
4). CRH acting as a neurotransmitter was found to play an integrative role across these domains in coordinating adaptive responses to stress. In addition to CRH, its two receptors (CRHR1 and CRHR2) were identified, as well as the CRHBP (
5,
6). Additional discoveries included the characterization of the urocortins, three additional endogenous ligands that are members of the CRH family with differing affinities for CRHR1 and CRHR2. All of the components of the CRH system are differentially but strategically located throughout the brain such that they are positioned to modulate the neural circuitry associated with stress and coping responses. Identification of the CRH receptors led to the development of numerous small-molecule antagonists aimed at targeting the CRHR1 receptor. Unfortunately, after numerous clinical trials, these antagonists were not found to be effective for the treatment of depression or anxiety disorders (
7). In the face of substantial preclinical data suggesting probable efficacy, it remains unclear why this strategy has not been successful. In part, the lack of efficacy of CRHR1 antagonists may be due to the capacity of the other components of the CRH system, including CRHBP, to rapidly adapt when perturbed. This suggests the possibility that targeting more than one of the CRH system components may be a more effective treatment strategy. Despite efforts, no small-molecule antagonists selective for CRHR2 have been identified and no strategies aimed at altering CRHBP have been explored for clinical utility.
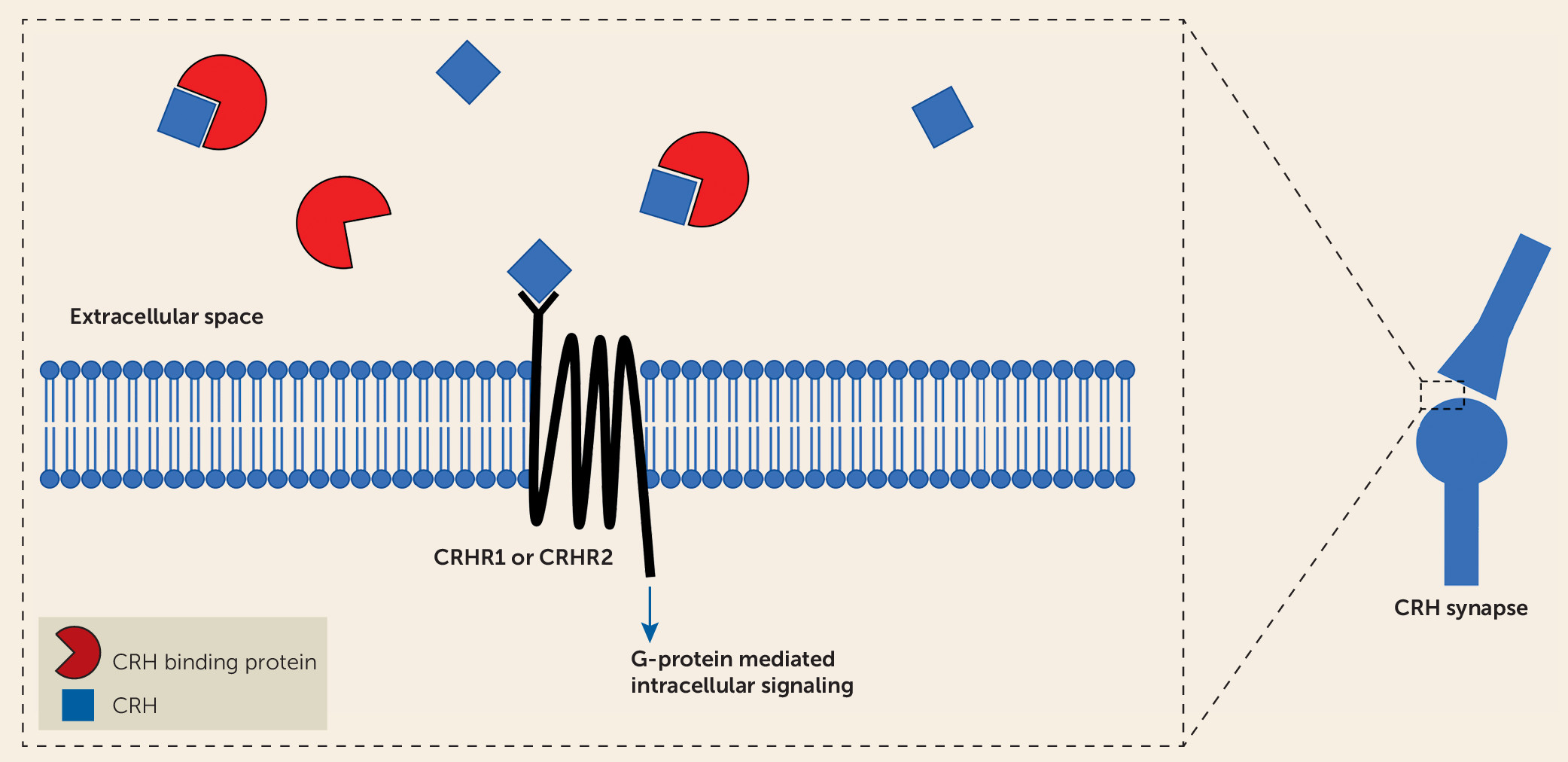
The findings reported by O’Connell et al. point to a potentially important role of CRHBP in modulating SSRI antidepressant treatment response and also suggest that alterations in CRHBP function could be important in the pathophysiology of stress-related psychopathology. CRHBP was originally isolated from human plasma and is hypothesized to function as a secreted protein modulating the actions of CRH (
8). Subsequently, CRHBP was found to be expressed in the brain, the pituitary, and other peripheral tissues. Notably, CRHBP is expressed in key brain regions implicated in the pathophysiology of anxiety and depression, including the hippocampus, the ventral tegmental area, the bed nucleus of the stria terminalis, and the central nucleus of the amygdala (
6,
8). CRHBP is thought to function by sequestering CRH, thereby reducing its availability to activate CRH receptors (
Figure 1). However, some studies indicate additional roles for CRHBP. For example, data suggest that CRHBP may facilitate the actions of CRH at CRHR2 receptors in the ventral tegmental area. In addition, CRHBP has been suggested to enhance CRH signaling through CRH receptor–independent mechanisms and potentially to aid in the trafficking of the CRHR2 to the cell surface (
8). Numerous studies in rodents have shown that levels of CRHBP in various brain regions are highly responsive to psychological stressors (see reference
8 for a review). Additionally, one CRHBP SNP was previously associated with treatment response (differing from the SNP associated with treatment response reported in the O’Connell et al. study), and two additional SNPs have been associated with depression (
9). Finally, Herringa et al. (
10) reported a nonsignificant decrease in CRHBP mRNA levels in postmortem amygdala tissue in males suffering from major depression, and significant decreases in CRHBP expression in males with schizophrenia and bipolar disorder.
Together, this evidence makes CRHBP an interesting candidate in conceptualizing mechanisms by which acute and long-term stress influence the development of psychopathology. From the findings of the O’Connell et al. report, it appears that genetic variation in CRHBP may be involved with mediating treatment response to SSRIs and may serve to inform antidepressant treatment selection. As the authors point out, if these findings stand the test of time, they may be useful from a “personalized medicine” perspective to enhance the likelihood of successful antidepressant treatment. Finally, these and other data suggest that it is reasonable to consider the development of new antidepressant treatment strategies focused on modulating CRHBP function.