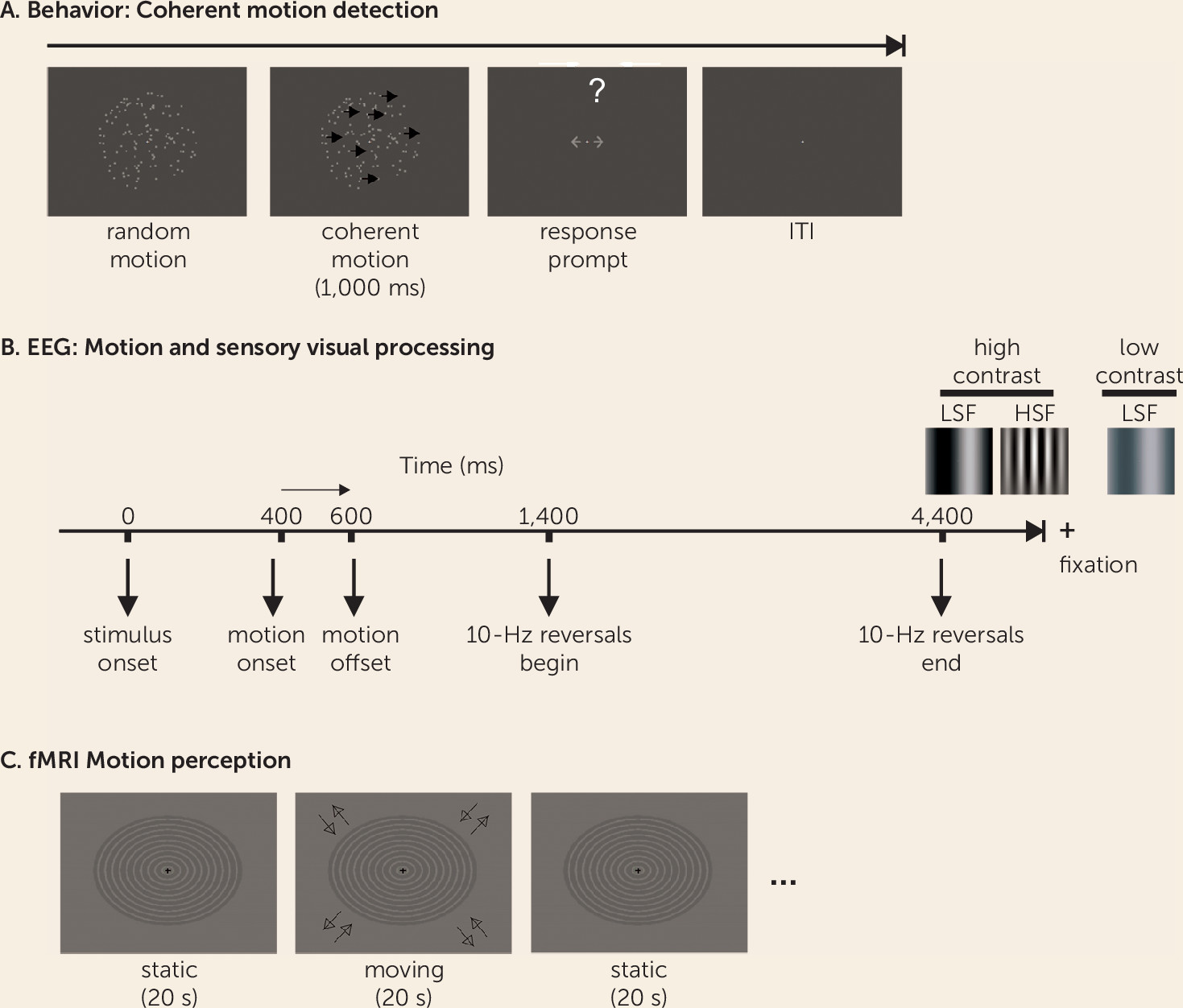
The ability to perceive and process motion is central to everyday human functioning and contributes strongly to an individual’s ability to decode the emotion and intent of another person on the basis of facial expression (face emotion recognition) (
1,
2). Motion discrimination ability is known to be heavily dependent on the function of visual sensory regions located in the mid-temporal cortex, which can be investigated physiologically using electrophysiological (
3) and functional MRI (fMRI) approaches (
4). In addition to its role in simple motion processing, the mid-temporal cortex area plays an important role in decoding facial expressions, suggesting that the decoding of emotion, even from static faces, may depend in part on processing of the implied motion inherent in the facial display (
5).
Deficits in motion detection ability were first demonstrated in schizophrenia about 20 years ago (
6). However, determination of the course of such deficits and identification of their underlying neural mechanisms remain areas of active investigation. Here, we investigated motion processing ability, along with other aspects of visual function, both in patients with schizophrenia and in patients who met criteria for attenuated psychosis syndrome, which is included in DSM-5 (
7) as a condition for further study. In addition to showing attenuated symptoms of schizophrenia, individuals with attenuated psychosis syndrome show significant impairments in social and role functioning and markedly elevated risk for transitioning to schizophrenia (
8).
In primates, the visual sensory cortex receives convergent information from several distinct subcortical pathways. The magnocellular pathway is uniquely sensitive to low-contrast, low-spatial-frequency stimuli, and stimulus motion. In contrast, the parvocellular system is specialized for the processing of high-spatial-frequency and high-contrast information (
9). The retinotectal system, which projects from the retina to the superior colliculus and then to the pulvinar nucleus of the thalamus, is proposed to account for the phenomenon of blindsight following lesions in the primary visual cortex as well as for rapid activation in the amygdala in response to fearful faces (for a review, see reference
10).
Consistent with motion processing dysfunction, robust deficits in magnocellular function have been documented in schizophrenia using behavioral, electrophysiological, and fMRI methods and have been linked to underlying dysfunction of
N-methyl-
d-aspartate-mediated glutamatergic neurotransmission (for a review, see references
11–
13). Impairments in visual processing are also concordant with postmortem structural studies that show consistent reductions of volume and neuron number in both the primary visual cortex (
14) and the pulvinar (
15). Occipital volumes are apparently unaffected in individuals with attenuated psychosis as a whole (
16) but may differentiate individuals who transition to schizophrenia from those who do not (
17).
Here, we analyzed electrophysiological responses derived from an optimized visual stimulation paradigm (which we refer to as the “JH-FLKR” paradigm) in patients with schizophrenia and patients with attenuated psychosis. Spectral decomposition (time frequency) analysis was used to isolate components of interest. In this paradigm, oscillatory activity related to initial stimulus onset occurs primarily at theta frequency (4–7 Hz) (
18). By contrast, motion onset has been shown to evoke slower activity that mapped to the delta frequency range (1–4 Hz) (
19). Steady-state visual evoked potentials elicited by alpha frequency (10 Hz) oscillatory stimuli were also measured. Steady-state visual evoked potentials have been shown to be reliably reduced in schizophrenia (for a review, see reference
20) but have not been studied in attenuated psychosis. In the present study, we obtained fMRI measures of motion processing from a subset of patients with schizophrenia.
Our aim was twofold: first, to investigate the integrity of visual processing dysfunction across two clinical groups—patients with schizophrenia and patients with attenuated psychosis—and second, to examine functional correlates of visual sensory impairments. Given recent findings that deficits in face emotion recognition precede illness onset and predict transition to schizophrenia (
21,
22), we hypothesized that deficits in motion processing would likewise predate illness onset and correlate with impaired face emotion recognition and other aspects of cognitive processing in patients with schizophrenia and in patients with attenuated psychosis.
Results
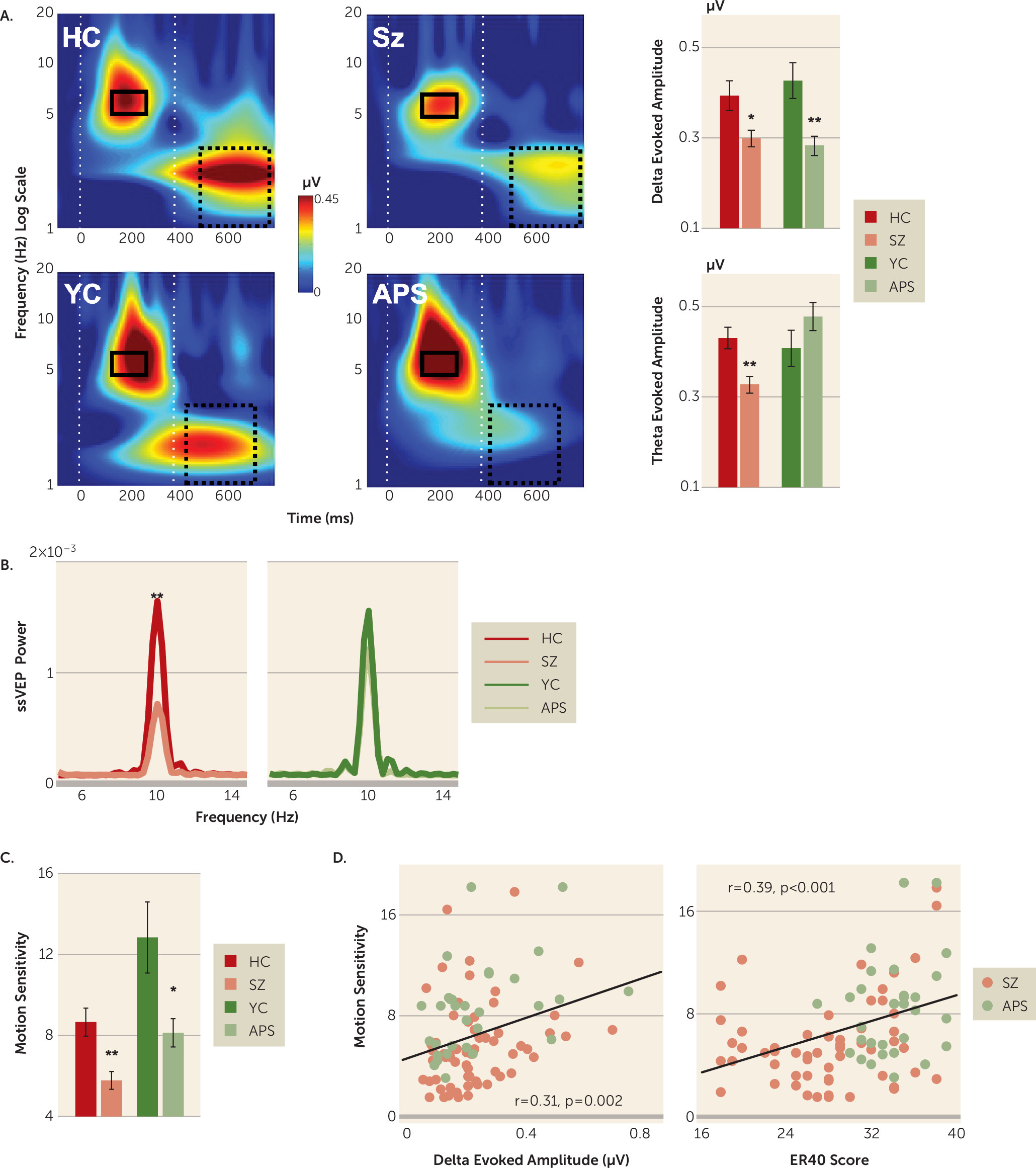
Time-frequency plots by clinical status (schizophrenia and attenuated psychosis) and age group (older healthy control group and younger healthy control group) are shown in
Figure 2. Separate analyses were conducted for delta, theta, and steady-state visual evoked potential responses.
Motion-Evoked (Delta) Activity
Motion-related delta activity differed significantly between clinical subjects and healthy control subjects (F=17.66, df=1, 152, p<0.001, d=0.68), with no significant main effect of age group (F=0.006, df=1, 152, p=0.938, d=0.02) or clinical status-by-age-group interaction (F=0.779, df=1, 152, p=0.379). Highly significant between-group differences were observed for both the schizophrenia group (t=2.71, df=105, p=0.008, d=0.54) and the attenuated psychosis group (t=3.45, df=53, p=0.001, d
=0.96) compared with age-matched healthy control subjects (schizophrenia group compared with older control subjects and attenuated psychosis group compared with younger control subjects) (
Figure 2A; for further details, see Table S3 in the
online supplement).
There was also a significant stimulus type-by-clinical status interaction (F=3.45, df=2, 151, p=0.034), reflecting greater deficits for low spatial frequency stimuli of both high-luminance contrast (t=4.50, df=160, p<0.001, d=0.72) and low-luminance contrast (t=4.50, df=160, p<0.001, d=0.53) compared with high-spatial-frequency stimuli (t=4.50, df=160, p<0.001, d=0.43), supporting differential magnocellular system involvement.
Finally, there was a small effect size (nonsignificant effect of testing site [F=3.75, df=1, 152, p=0.055, d=0.31]) that did not significantly interact with clinical status (F=0.012, df=1, 152, p=0.915) or age group (F=0.221, df=1, 152, p=0.639).
Stimulus-Onset (Theta) Activity
In contrast with motion-onset responses, stimulus-onset (theta) responses were not significantly different between clinical subjects and healthy control subjects overall (F=0.27, df=1, 152, p=0.607, d=0.08). However, there was a highly significant clinical status-by-age group interaction (F=9.14, df=1, 152, p=0.003), reflecting significantly reduced mean theta activity in patients with schizophrenia compared with older healthy control subjects (t=3.48, df=105, p<0.001, d=0.69) but preserved stimulus-onset responses in patients with attenuated psychosis (t=–1.41, df=53, p=0.164, d=0.39) (
Figure 2A).
The effect of age group was significant (F=4.99, df=1, 152, p=0.027, d=0.36), reflecting larger responses in younger individuals compared with older individuals irrespective of their clinical status. The stimulus type-by-clinical or type-by-nonclinical interaction was not statistically significant (F=0.757, df=2, 151, p=0.470). There was no main effect of testing site (F=0.013, df=1, 152, p=0.911, d=0.02), site-by-age group interaction (p=0.173), or site-by-clinical status interaction (p=0.898) (for further details, see Table S3 in the online supplement).
Steady-State Visual Evoked Potentials (Fast Fourier Transform) Power (Alpha)
As with theta, power for steady-state visual evoked potential was not significantly different between the patient and healthy control groups overall (F=2.20, df=1, 152, p=0.140, d=0.24) but did show a significant clinical status-by-age group interaction (F=6.20, df=1, 152, p=0.014), reflecting significant deficits in the schizophrenia group (t=3.16, df=105, p=0.002, d=0.63) but not in the attenuated psychosis group (t=–0.68, df=53, p=0.495, d=0.19) compared with age-matched control subjects (
Figure 2B). The main effect of testing site (F=0.007, df=1, 152, p=0.932, d=0.01) and all interactions with testing site were nonsignificant (all p values >0.25).
Motion Sensitivity (Behavior)
As with delta, behavioral motion sensitivity (1/coherence threshold) differed significantly between clinical subjects and control subjects (F=20.02, df=1, 152, p<0.001, d=0.72), reflecting significant reductions both in patients with schizophrenia (p<0.001) and in patients with attenuated psychosis (p<0.01) compared with age-matched control subjects (
Figure 2C). There was a significant main effect of age group (F=17.401, df=1, 152, p<0.001, d=0.66), reflecting better performance among younger individuals compared with older individuals; however, clinical status and age did not significantly interact (F=1.05, df=1, 152, p=0.305).
Although motion sensitivity differed as a function of testing site (F=5.03, df=1, 152, p=0.026, d=0.36), there was no interaction between site and clinical status (F=0.273, df=1, 152, p=0.602) or site and age (F=2.32, df=1, 152, p=0.130).
Across the schizophrenia and attenuated psychosis groups, the amplitude of both delta-evoked (r=0.309, p=0.002) and theta-evoked activity (r=0.290, p=0.004) correlated significantly with impaired motion sensitivity. Reduced motion sensitivity in the two clinical groups additionally correlated with impaired face emotion recognition (Penn Emotion Recognition Task) (r=0.385, p<0.001) (
Figure 2D).
fMRI
During scanning, correct detections of fixation dimming events did not differ significantly between patients with schizophrenia (79%) and healthy control subjects (87%) (t=0.57, df=35, p=0.581, d=0.19).
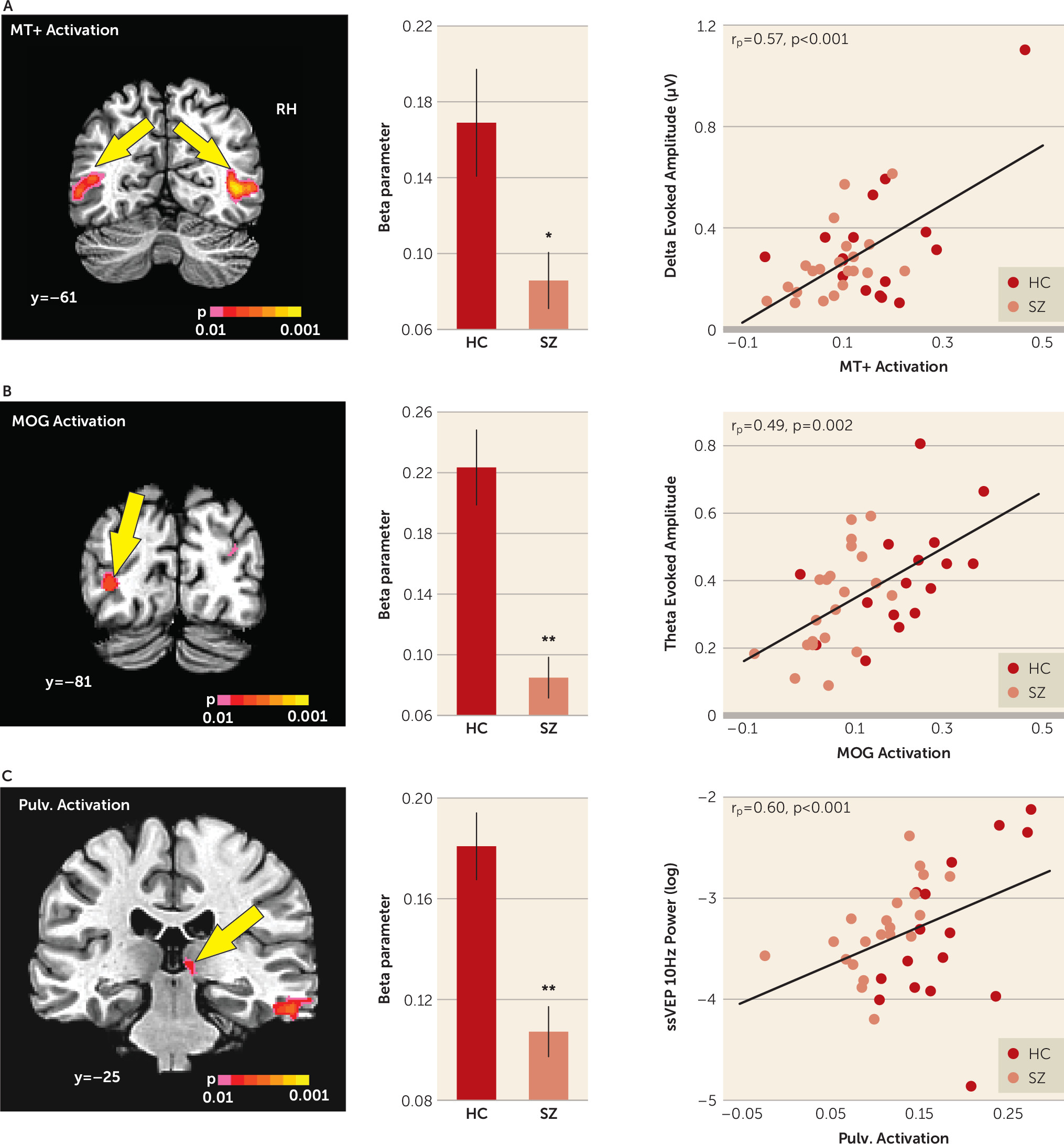
Activation in the mid-temporal cortex in response to moving compared with stationary stimuli was reduced in patients with schizophrenia overall (F=6.54, df=1, 35, p=0.015, d=0.87). The main effect of hemisphere was also significant (F=18.09, df=1, 35, d=1.45, p=0.001), with greater activation in the right hemisphere. However, the group-by-hemisphere interaction was not statistically significant (F=0.757, df=1, 35, p=0.390). Finally, the mean activation in the mid-temporal cortex correlated significantly with motion-evoked delta activity across all groups (r
p=0.573, p<0.001) and within the schizophrenia group (r=0.642, p=0.002) and the healthy comparison group (r=0.583, p=0.018) (
Figure 3A).
Significant main effects of group were observed in the middle occipital gyrus in the left hemisphere (F=27.44, df=1, 35, p<0.001, d=1.79), which correlated with reduced mean theta amplitude across groups (r
p=0.493, p=0.002) and within groups (schizophrenia group: r=0.485, p=0.026; control groups: r=0.518, p=0.040) (
Figure 3B). Significant main effects of group were also observed in the right pulvinar of the thalamus (F=20.62, df=1, 35, p<0.001, d=1.55), which correlated with reduced steady-state visual evoked potential power both in schizophrenia patients (r=0.511, p=0.018) and in healthy comparison subjects (r=0.740, p=0.001) as well as across groups (r
p=0.60, p<0.001) (
Figure 3C).
Reduced activations were observed in additional occipital and frontal brain regions (for further details, see Table S4 in the online supplement). However, no significant correlations were observed with any electrophysiological measures for these regions in any group (all p values >0.25).
Correlations With Neurocognitive Measures
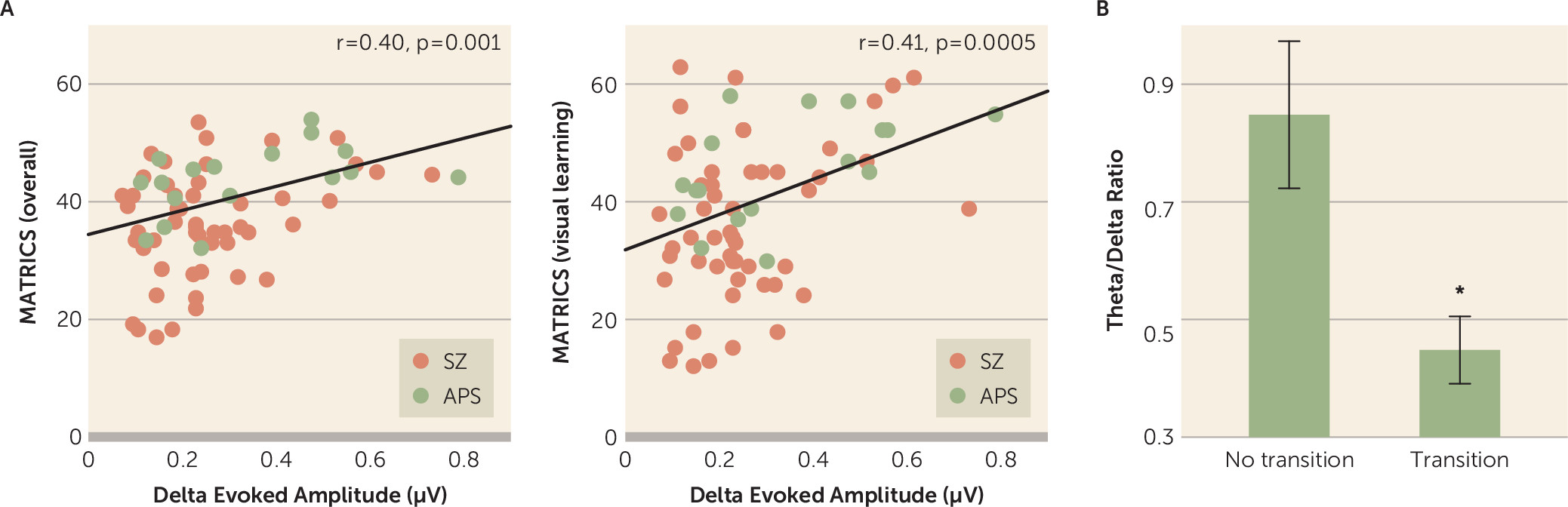
Across the two patient groups (schizophrenia and attenuated psychosis), reduced motion-evoked delta activity correlated with lower overall composite scores on the MCCB (r=0.397, p=0.001) (
Figure 4A) as well as lower scores on the visual learning domain (r=0.408, p<0.001) (
Figure 4A), the attention/vigilance domain (r=0.271, p=0.024), and the speed of processing domain (r=0.392, p=0.001). Similarly, mean theta amplitude evoked by stimulus onset correlated with scores on the domains of visual learning (r=0.296, p=0.014) and processing speed (r=0.406, p=0.001) across all patients.
Transition to Schizophrenia
Of the 32 patients with attenuated psychosis, six transitioned to psychosis (all to schizophrenia) over a 2-year follow-up period. Among patients with attenuated psychosis, the ratio between delta and theta activity differed significantly between individuals who did and did not transition to schizophrenia (t=2.91, df=30, p=0.007, d=1.06) (
Figure 4B), with a cutoff value of 0.7 (equivalent to a 99% confidence interval for the remaining participants) correctly predicting 100% of those who did transition but excluding 35% (N=9/26) of those who did not transition (likelihood ratio χ
2=4.48, p=0.034).
Furthermore, across patients with attenuated psychosis, the delta and theta ratios correlated significantly with positive (r=−0.32, p=0.036), negative (r=−0.32, p=0.036), and general (r=−0.33, p=0.029) symptoms as ascertained using the Scale of Prodromal Symptoms. Finally, face emotion recognition scores (ascertained with the Penn Emotion Recognition Task) also significantly differentiated individuals who transitioned to schizophrenia from those who did not (t=2.25, df=30, p=0.045, d=0.82). By contrast, no significant differences were observed for either neurocognitive measures (ascertained with the MCCB) or symptom measures (ascertained with the Scale of Prodromal Symptoms).
Control Analyses
When separate ANOVAs were conducted by testing site, both motion-evoked delta activity (Nathan Kline Institute for Psychiatric Research: p=0.001; New York State Psychiatric Institute: p=0.008) and behavioral motion-sensitivity (Nathan Kline Institute for Psychiatric Research: p<0.001; New York State Psychiatric Institute: p=0.026) were independently significant for patients with schizophrenia or attenuated psychosis compared with age-matched control subjects at each site. For theta (Nathan Kline Institute for Psychiatric Research: p=0.011; New York State Psychiatric Institute: p=0.01) and steady-state visual evoked potential power (Nathan Kline Institute for Psychiatric Research: p=0.009; New York State Psychiatric Institute: p=0.018), differences between patients with schizophrenia and healthy control subjects were also independently significant at each site.
No significant differences by sex were observed either across or within sites. Additionally, no significant correlations were observed between any measure and medication dosage (assessed in chlorpromazine equivalents) (
30).
Discussion
This study investigated the integrity of early visual processing both in patients with schizophrenia and in patients with attenuated psychosis and its linkage to cognitive function. The findings confirm the hypothesis that deficits in motion perception are present even before the onset of schizophrenia and are associated with impaired activation in motion-sensitive visual cortex (mid-temporal cortex area). Moreover, deficits in motion processing correlate significantly with impairments in face emotion recognition as well as with multiple domains of cognition in patients with schizophrenia and attenuated psychosis (
Figure 2). Overall, these findings highlight the importance of sensory-level dysfunction in the personal experience and neuronal machinery not only in patients with schizophrenia (
31) but also in individuals with attenuated psychosis who are at high risk of transitioning to schizophrenia.
In contrast to deficits in motion processing, patients with attenuated psychosis showed preservation of other aspects of early visual function that were also impaired in the schizophrenia group, demonstrating functional differences as well as similarities between the schizophrenia and attenuated psychosis syndromes. Specifically, the theta frequency response to stimulus onset was not impaired in patients with attenuated psychosis but was significantly reduced in patients with schizophrenia and correlated with impaired activation in the middle occipital gyrus (
Figure 3B). Likewise, whereas the amplitude of the alpha frequency steady-state visual evoked potential in response to steady-state stimuli was intact in patients with attenuated psychosis, in patients with schizophrenia it was significantly diminished and correlated with reduced functional activation in the pulvinar nucleus (
Figure 3C). This finding is consistent with the known role of the pulvinar in the generation of occipital alpha rhythms (
32–
34) as well as with studies showing that lesions of the pulvinar produce schizophrenia-like positive symptoms and language impairments in otherwise healthy individuals (
35). Taken together, the discrepancy in deficit patterns between patients with attenuated psychosis and patients with schizophrenia suggests that further deterioration in visual cortical processing may occur during transition to schizophrenia, potentially leading to additional visual impairments and social cognitive decline.
Although sensory processing was once considered an intact simple function, research has increasingly demonstrated the importance of both auditory and visual sensory disturbances in the pathophysiology of schizophrenia. Impaired motion perception, in particular, has been associated with deficits in several perceptual and cognitive processes, including eye tracking, biological motion detection, and potential for theory of mind (for a review, see reference
1). The present findings further suggest that motion processing deficits are present and may contribute to disability, even in individuals with only attenuated psychotic symptoms.
Impaired motion sensitivity, measured behaviorally, correlated with neurophysiological (delta) measures of poor motion processing both in patients with attenuated psychosis and in patients with schizophrenia as well as with deficits in the ability to detect emotion based on facial expression (
Figure 2D), a deficit that we (
21) and others (
36) have previously shown to be highly predictive of transition to schizophrenia. In the present study, static faces were used to evaluate correlations between visual sensory function and face emotion recognition. Nonetheless, the results encourage development of validated dynamic emotion paradigms with greater ecological validity (
5) than that of currently available measures, such as the Penn Emotion Recognition Task, and further suggest that application of such batteries may help to differentiate individuals with attenuated psychosis or schizophrenia from healthy individuals. Finally, deficits in the neurophysiological response to motion onset predicted impaired scores on the MCCB domains of visual learning (
Figure 4A), processing speed, and attention/vigilance among patients in the attenuated psychosis and schizophrenia groups, suggesting a significant contribution to impaired present function. Visual learning, for example, is significantly correlated with employability among patients with schizophrenia (
37).
Consistent with the differential impairments in delta frequency responses compared with theta frequency responses in the attenuated psychosis group, the dissociation between these measures (delta and theta ratios) differed significantly between patients who transitioned to schizophrenia and those who did not (
Figure 4B). Although this finding requires replication in a larger sample, the effect size of the difference (d=1.06) compares favorably with that of previously described biomarkers, such as the duration (d=0.71) and frequency (d=0.32) of mismatch negativity (
38,
39). Moreover, other clinical measures, such as MCCB scores and symptoms, did not significantly predict conversion to schizophrenia in the present sample. Further studies are needed to evaluate the degree to which the predictive value of the delta and theta ratios adds to previously identified predictors, such as impairments in face emotion recognition, mismatch negativity, negative symptoms, and MRI-based measures.
In summary, visual as well as auditory sensory deficits have become increasingly established in schizophrenia, especially involving the ability to detect motion, which in turn affects both cognitive and emotion detection processes. We showed that such deficits are present even in individuals with an attenuated psychosis syndrome and contribute to functional impairments and symptoms. Although we have provided initial evidence that visual processing deficits may be predictive of transition to schizophrenia, our findings need to be confirmed in larger prospective cohorts.
Limitations
There are several limitations to this study. First, it was conducted across two recruitment sites, which may have introduced variance. Nevertheless, the main finding of reduced motion sensitivity and reduced motion-related delta activity across the two clinical cohorts (attenuated psychosis and schizophrenia) remained strongly significant, even when the sites were considered independently, suggesting relative cross-site reliability of the measure. Second, patients with schizophrenia were all receiving antipsychotic medication, which may have affected behavioral and neurophysiological measures. Nevertheless, no correlations with medication dosages were observed. Furthermore, the finding of equivalent deficits in motion processing both in at-risk patients with attenuated psychosis and in patients with schizophrenia argues strongly against a significant medication effect. Finally, given the small number of individuals who transitioned to psychosis, the prediction analyses must be considered exploratory. Nevertheless, effect sizes were comparable to those of other well-established measures, encouraging further confirmatory investigations.